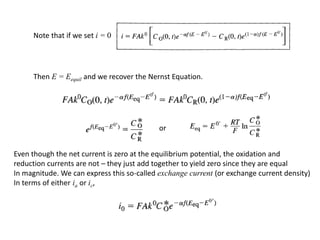
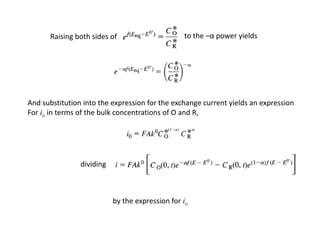
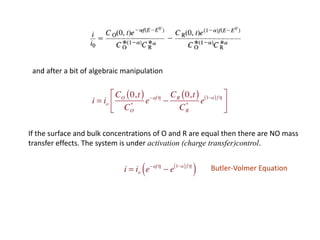
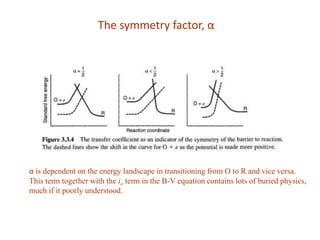
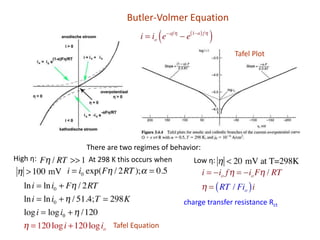
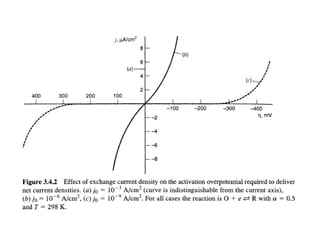
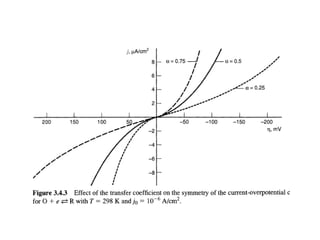
1. The document discusses electrode kinetics and the Butler-Volmer equation, which describes the relationship between current and overpotential during a redox reaction.
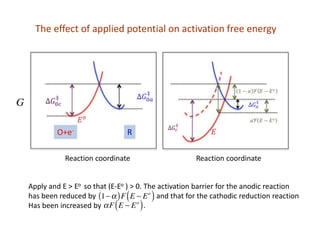
2. It explains how applying a potential above the equilibrium potential Eo lowers the activation barrier for oxidation and raises it for reduction.
3. The Butler-Volmer equation contains terms for the forward and backward rate constants that are dependent on the applied potential relative to Eo, as well as the exchange current density io which is proportional to the surface concentrations.
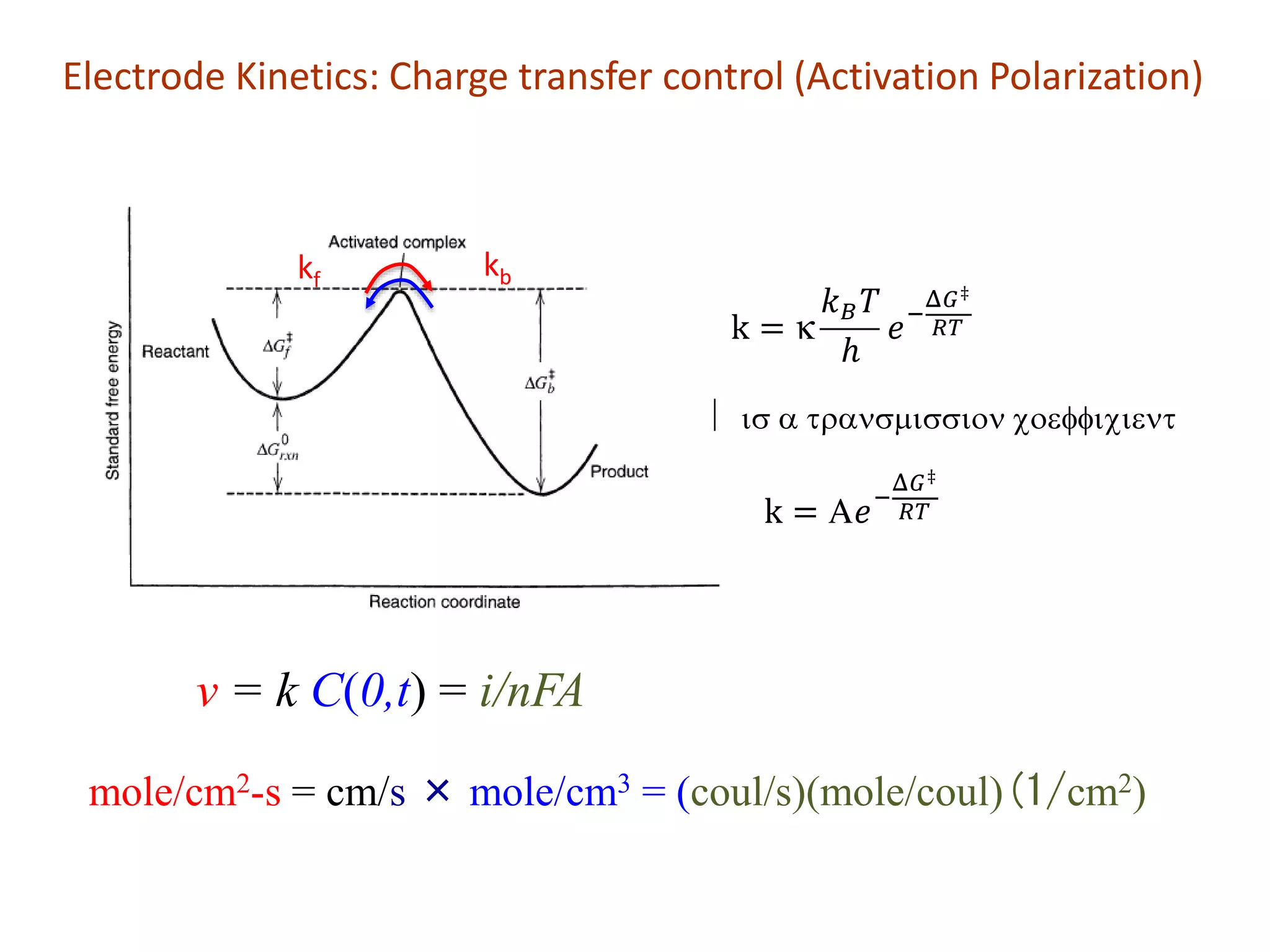
![v = kC(0,t) = i/nFA i = nFAkC(0,t)
inet = nFA[kf CO(0,t) - kbCR(0,t)]
vf = kf CO(0,t)
O + e- = R
vb= kbCR(0,t)
vnet=vf-vb = kf CO(0,t) - kbCR(0,t)
Since v is measured in moles/cm2-s if we multiply by the number of coulombs per mole
(nFA) in the rate equation we get a current.
Now consider a 1e- redox reaction
We can write a rate expression for
each and the net rate is just the
difference.](https://image.slidesharecdn.com/electrodekinetics-180804032955/85/Electrode-kinetics-2-320.jpg)

![Inserting these relations in to inet = nFA[kf CO(0,t) - kbCR(0,t)] yields,](https://image.slidesharecdn.com/electrodekinetics-180804032955/85/Electrode-kinetics-5-320.jpg)