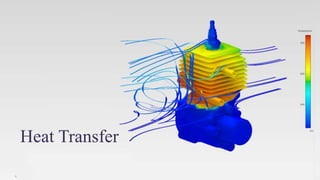
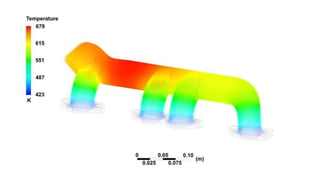
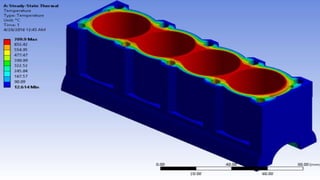
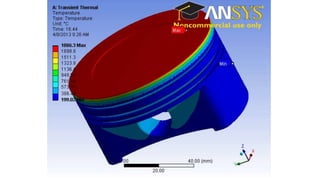
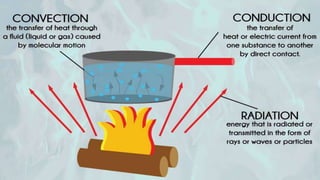
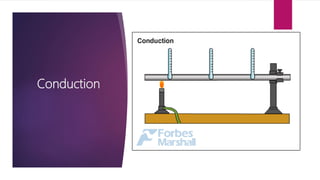
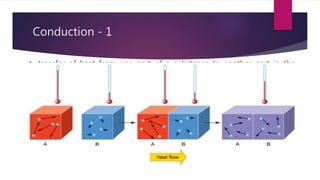
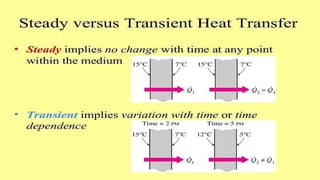
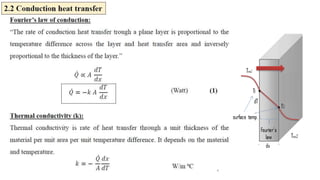
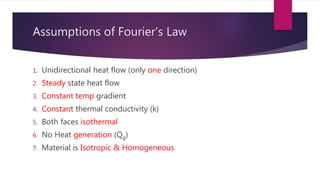
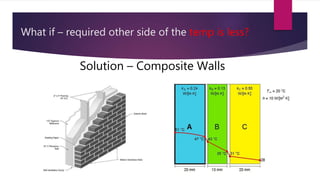
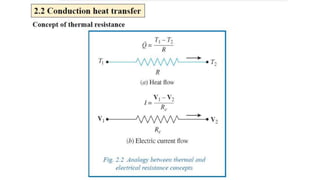
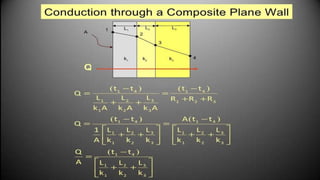
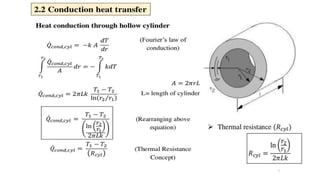
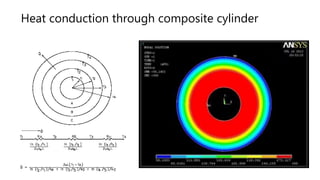
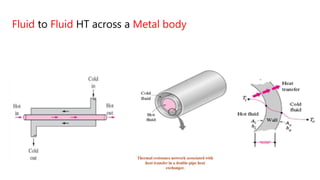
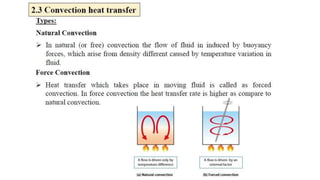
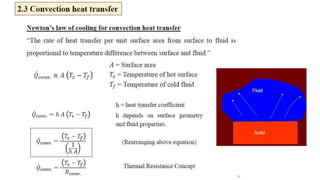
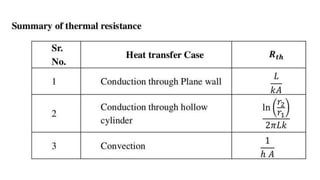
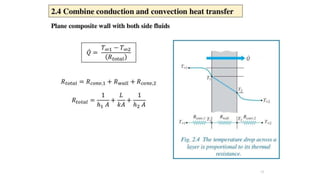
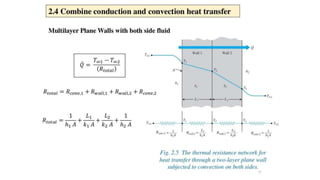
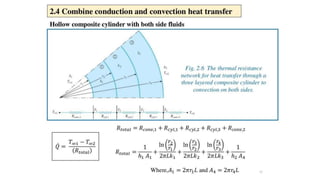
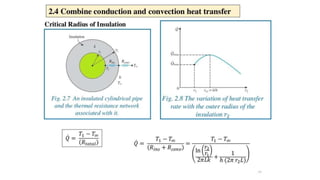
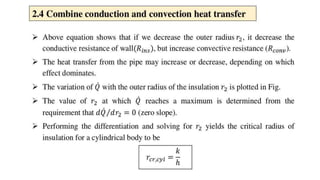
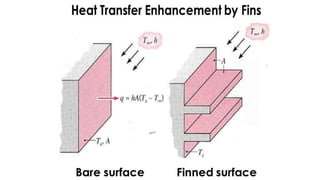
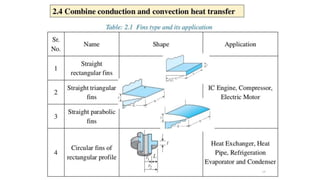
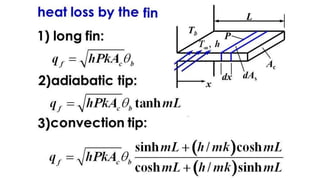
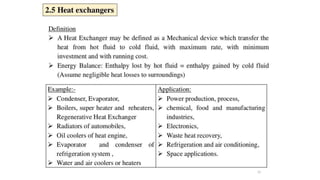
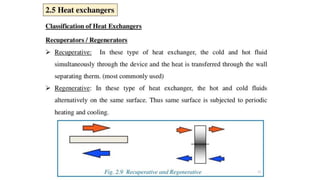
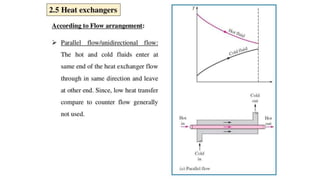
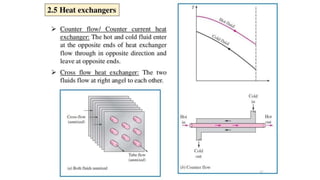
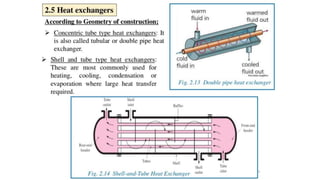
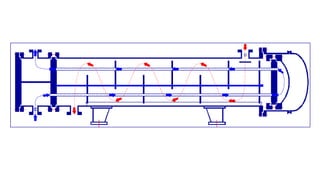
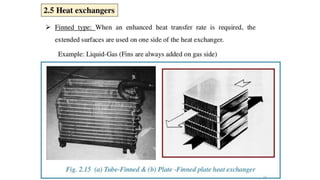
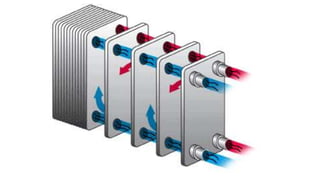
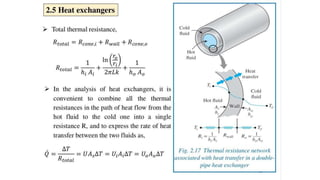
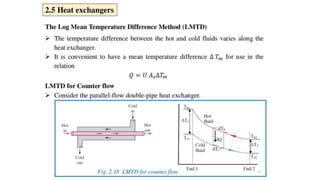
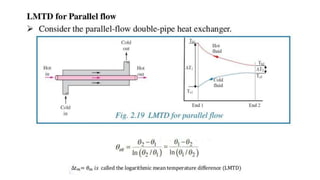
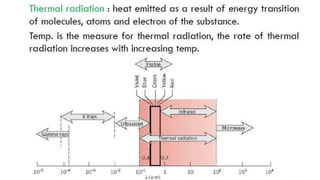
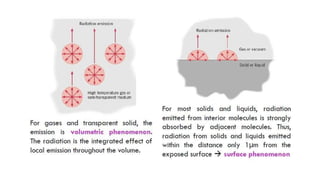
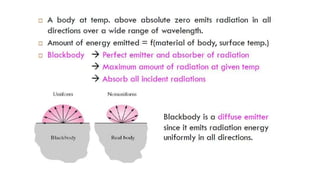
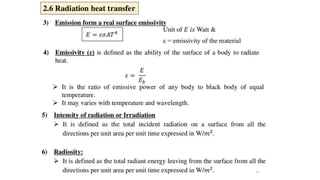
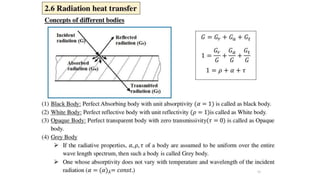
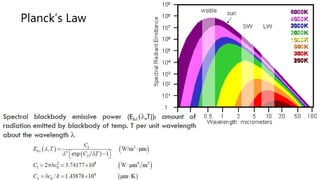
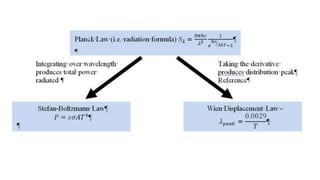
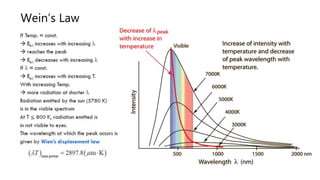
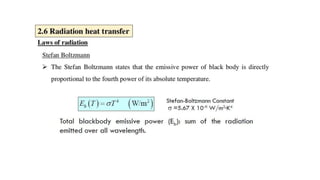
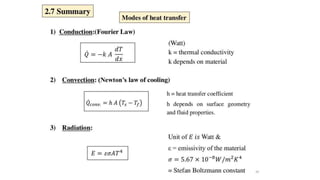
The document explains the concepts of heat transfer and thermodynamics, emphasizing that energy is the capacity to do work and heat energy is the transfer of energy due to temperature differences. It outlines the methods of heat transfer, primarily conduction, and distinguishes between thermal conductors and insulators. The document also illustrates practical applications of heat transfer principles in heating systems and various materials.