This document discusses various methods for water softening including:
1. Removal of temporary hardness can be done by boiling or adding lime to precipitate calcium carbonate.
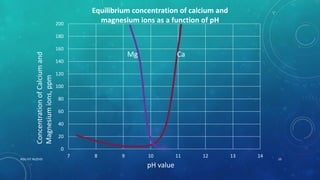
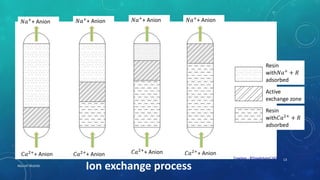
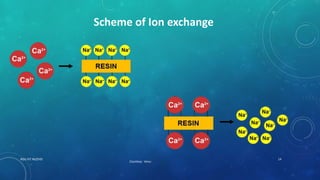
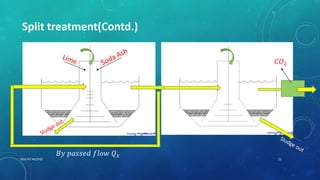
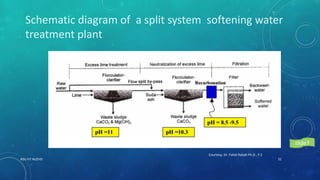
2. Permanent hardness can be removed through chemical precipitation using lime soda ash or ion exchange which replaces calcium and magnesium ions with sodium ions.
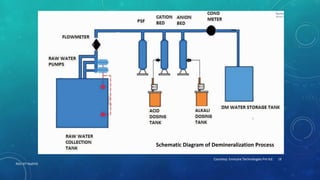
3. Demineralization passes water through cation then anion exchange resins to remove all minerals including hardness.