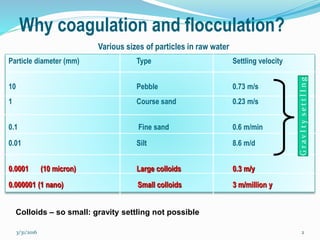
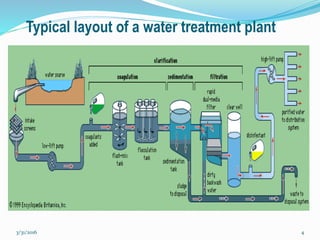
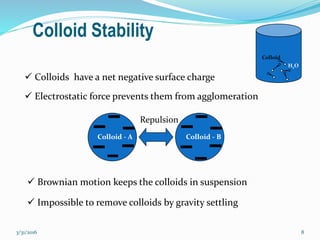
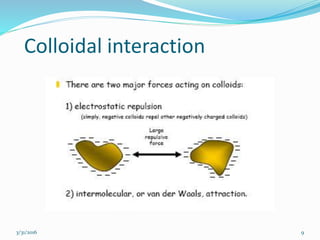
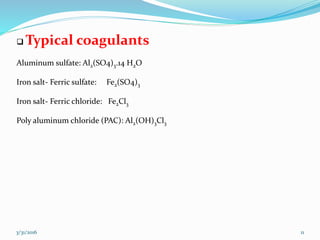
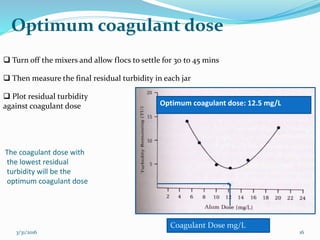
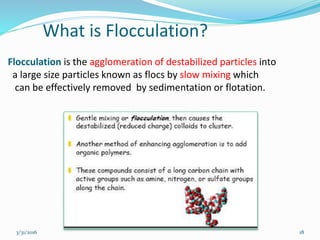
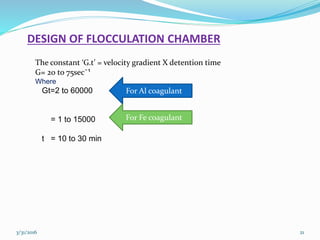
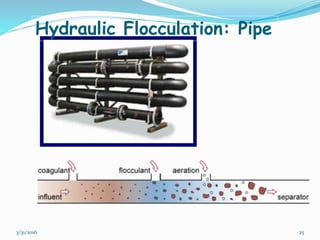
Coagulation and flocculation are important water treatment processes used to remove small particles from water. Coagulation involves adding chemicals like aluminum sulfate or ferric chloride to destabilize colloidal particles and reduce charges. This allows particles to agglomerate into larger flocs during flocculation. Jar tests are used to determine the optimum pH and coagulant dose. Mechanical and hydraulic flocculators are then used to slowly mix water and form flocs, which are removed by sedimentation. Proper design of coagulant chambers, flocculators, and clarifiers is needed for effective treatment.