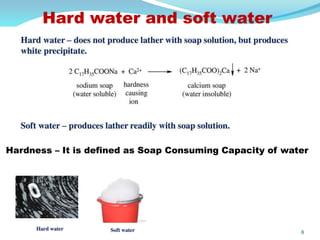
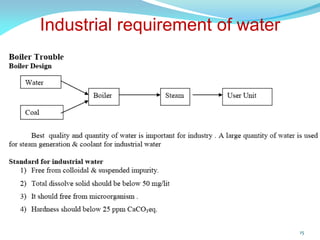
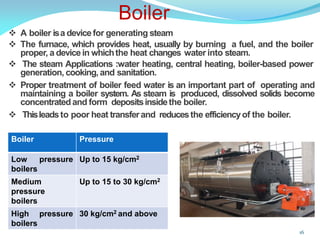
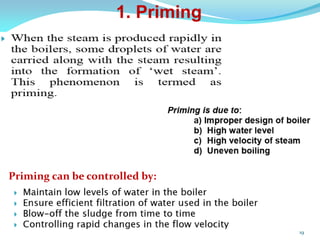
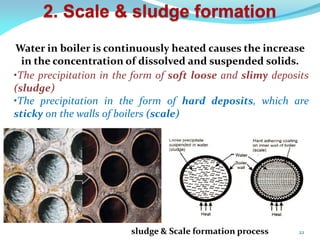
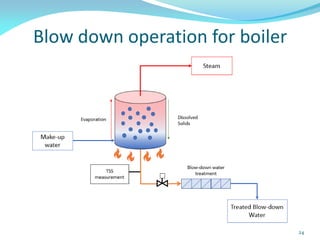
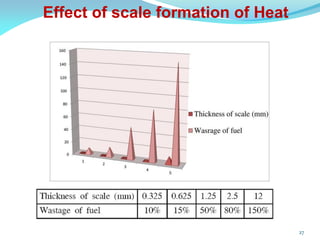
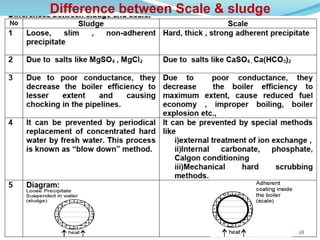
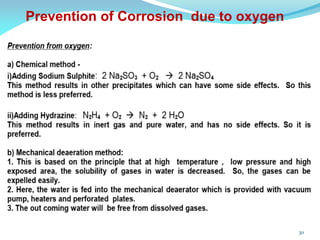
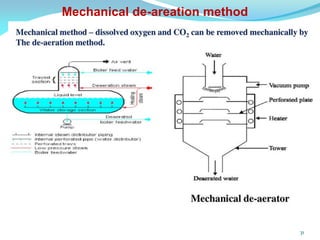
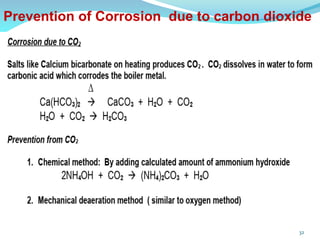
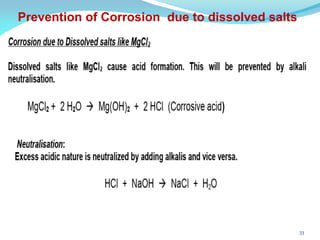
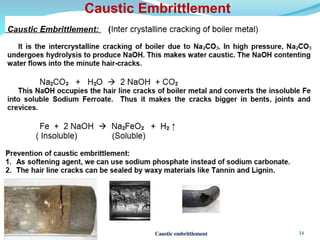
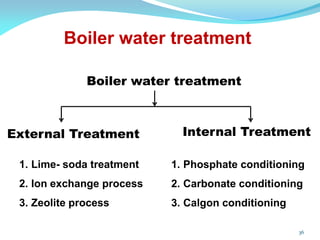
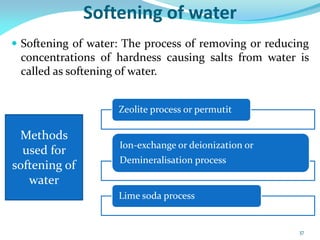
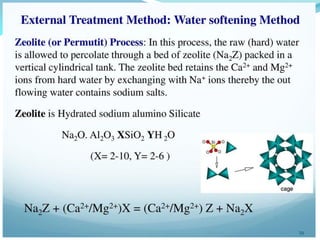
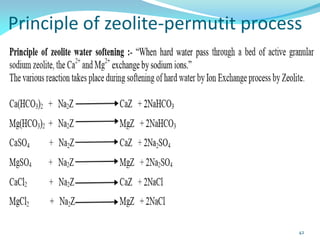
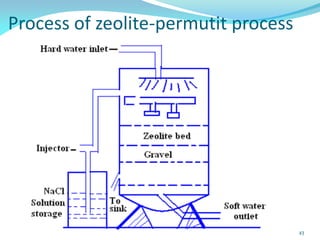
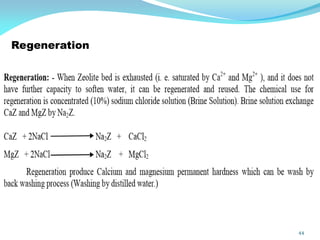
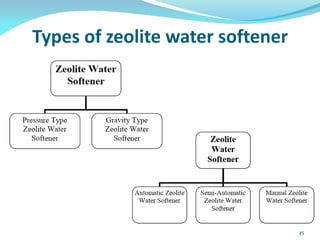
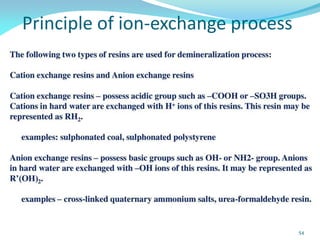
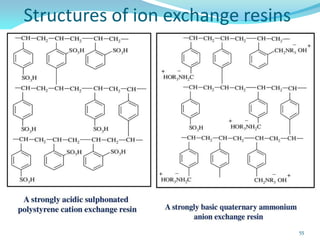
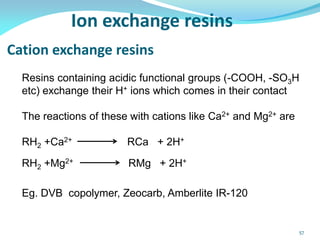
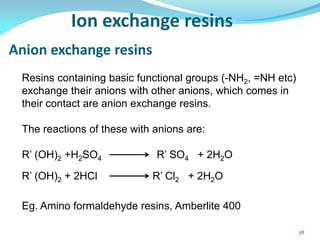
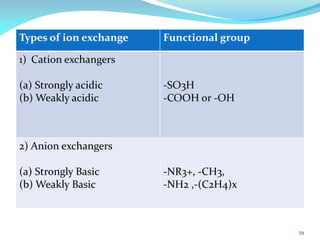
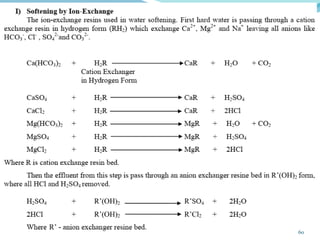
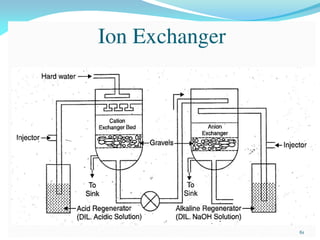
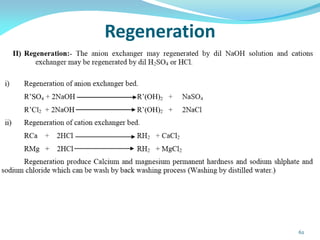
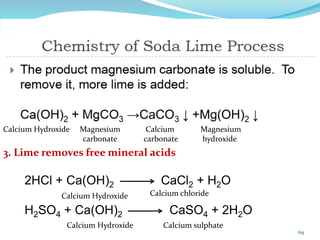
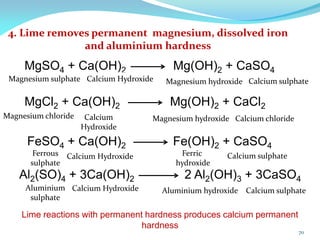
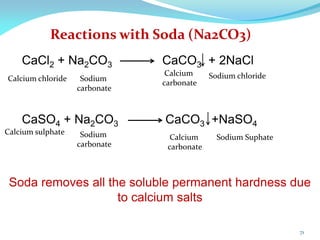
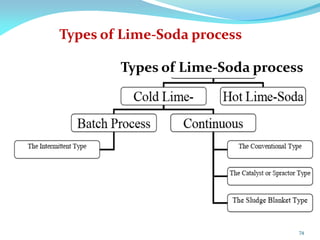
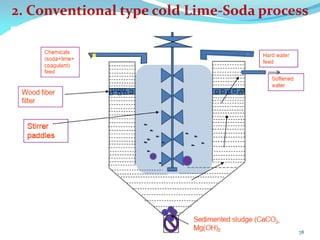
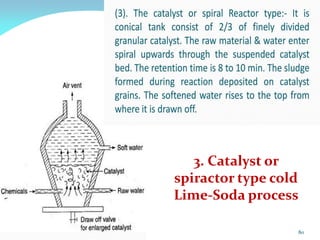
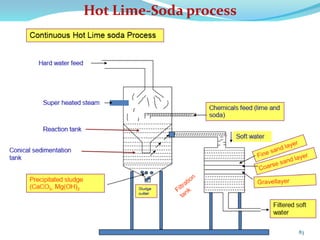
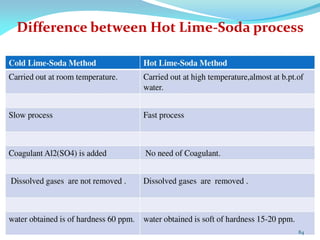
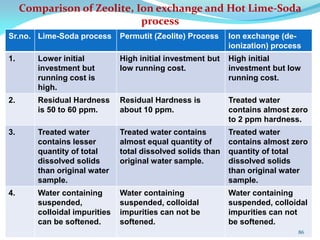
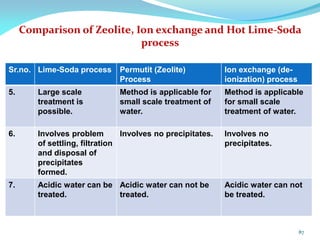
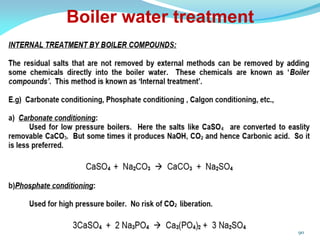
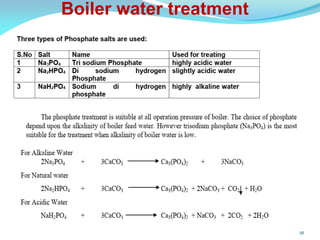
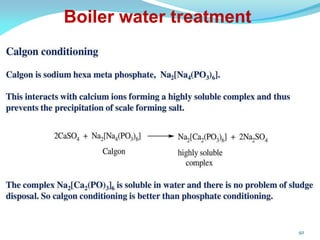
This document discusses various water treatment methods. It begins by introducing hard water and soft water, and the requirements and issues with boiler feed water. It then describes several external and internal water softening methods, including lime-soda, zeolite, and ion exchange. Lime-soda involves using lime and soda ash to precipitate hardness ions from water. Zeolite and ion exchange use ion exchange resins to remove hardness. The document provides details on the principles, processes, equipment used and regeneration for each method. It also discusses other water treatment issues like boiler troubles, scale formation, corrosion prevention and domestic water treatment.