1. Water is essential for human survival but is often contaminated.
2. Water sources include surface water and groundwater, with varying levels of physical, chemical, and biological impurities depending on the source.
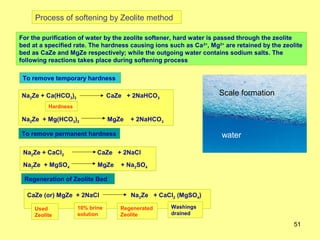
3. Hard water contains high levels of calcium and magnesium ions which can cause soap scum, clog pipes and boilers, and is classified as temporary or permanent hardness. Preventing scale formation in boilers is important to improve efficiency and avoid accidents.







![pH
• pH a measure of hydrogen ion activity is used to
express the intensity of acidic or alkaline condition of a
solution.
• The pH scale runs to 0 from 14 with 0 representing
maximum acidity and 14 maximum basicity
pH = -log [H+]
8](https://image.slidesharecdn.com/unit1watertechnology-121111071552-phpapp01/85/Unit-1-water_technology-8-320.jpg)



















![3. Calgon conditioning
Na 2 [Na 4 (PO 3 ) 6 2Na+ +
[Na 4 P 6 O 18 ] 2-
Calgon – sodium
hexa meta
phosphate
2CaSO 4 (Boiler water) + [Na 4 P 6 O 18 ] 2- [Ca 2 P 6 O 18 ] 2- + 2Na 2 SO 4
Calcium
Soluble complex ion
sulfate
of calcium - can be
removed easily
Calgon tablets are used in the cleaning of washing machine
drums
28](https://image.slidesharecdn.com/unit1watertechnology-121111071552-phpapp01/85/Unit-1-water_technology-28-320.jpg)


![IV. Boiler
corrosion
Degradation or destruction of boiler materials (Fe) due to the
chemical or electrochemical attack of dissolved gases or salts is
called boiler corrosion
Boiler corrosion is of three types
1. Corrosion due to dissolved O 2
2. Corrosion due to dissolved CO 2
3. Corrosion due to acids formed by dissolved
salts
1. Corrosion due to dissolved oxygen
(DO)
2 Fe + 2 H2O + O2 2 Fe(OH)2
4 Fe(OH)2 + O2 2 [Fe2O3.2H2O]
Ferrous Rust
hydroxide
31](https://image.slidesharecdn.com/unit1watertechnology-121111071552-phpapp01/85/Unit-1-water_technology-31-320.jpg)