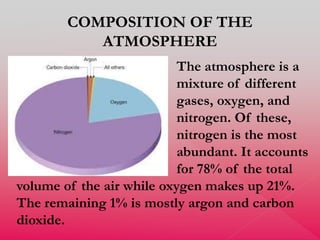
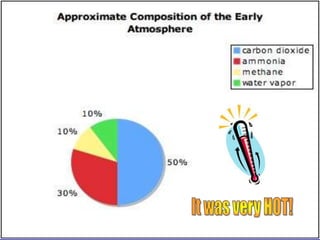
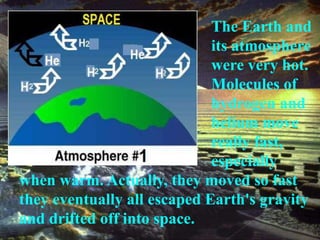
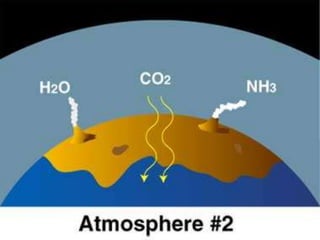
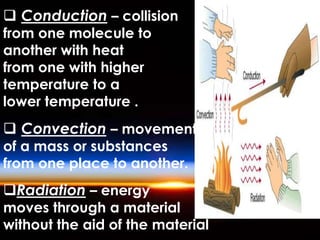
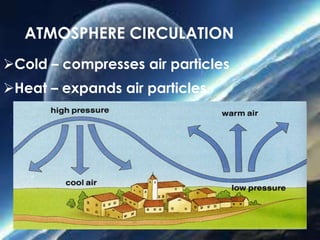
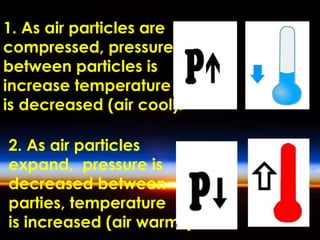
The document discusses the composition and evolution of Earth's atmosphere. It notes that nitrogen makes up 78% of the atmosphere, while oxygen is 21% and other gases like argon and carbon dioxide make up the remaining 1%. Earth originally had an atmosphere of hydrogen and helium that eventually escaped. Later, volcanoes released water vapor, carbon dioxide, and ammonia, forming a second atmosphere. Cyanobacteria introduced oxygen to the atmosphere between 3.3 to 2.2 billion years ago. The modern atmosphere of nitrogen and oxygen is considered the third atmosphere and has a composition influenced by biological and geological processes.