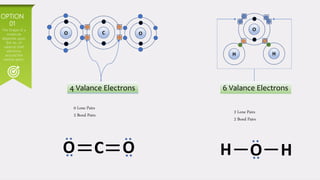
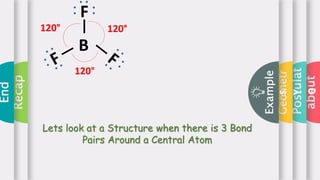
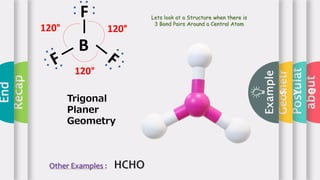
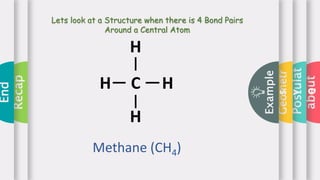
This document provides an overview of Valence Shell Electron Pair Repulsion (VSEPR) theory. It begins with an introduction and outlines the theory's postulates. Next, it discusses molecular geometry, presenting examples that illustrate linear, trigonal planar, tetrahedral, and bent geometries. The document concludes with a recap of the key aspects of VSEPR theory covered.