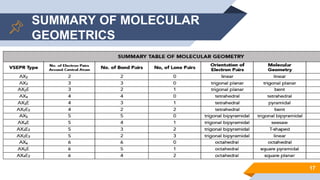
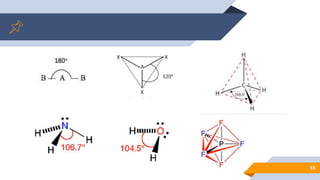
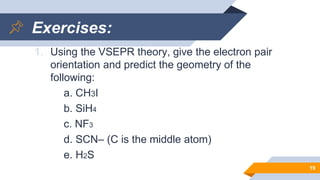
The document discusses molecular geometry and polarity, focusing on predicting molecular shapes using the valence shell electron pair repulsion (VSEPR) theory. It outlines key concepts such as bond angles, dipole moments, and the classification of molecular shapes based on electron pair orientations. Exercises are provided to apply the learned concepts on specific molecules.