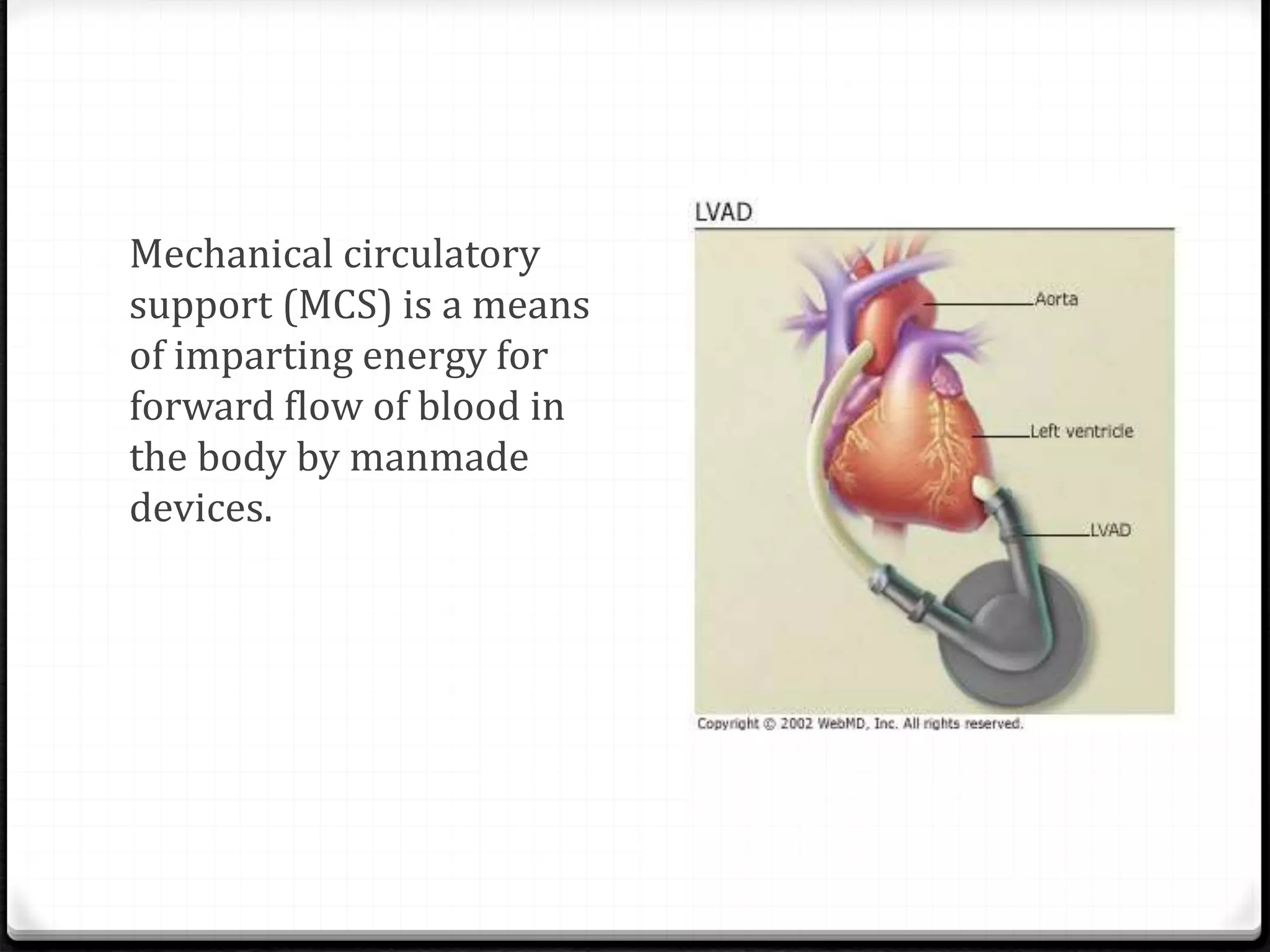
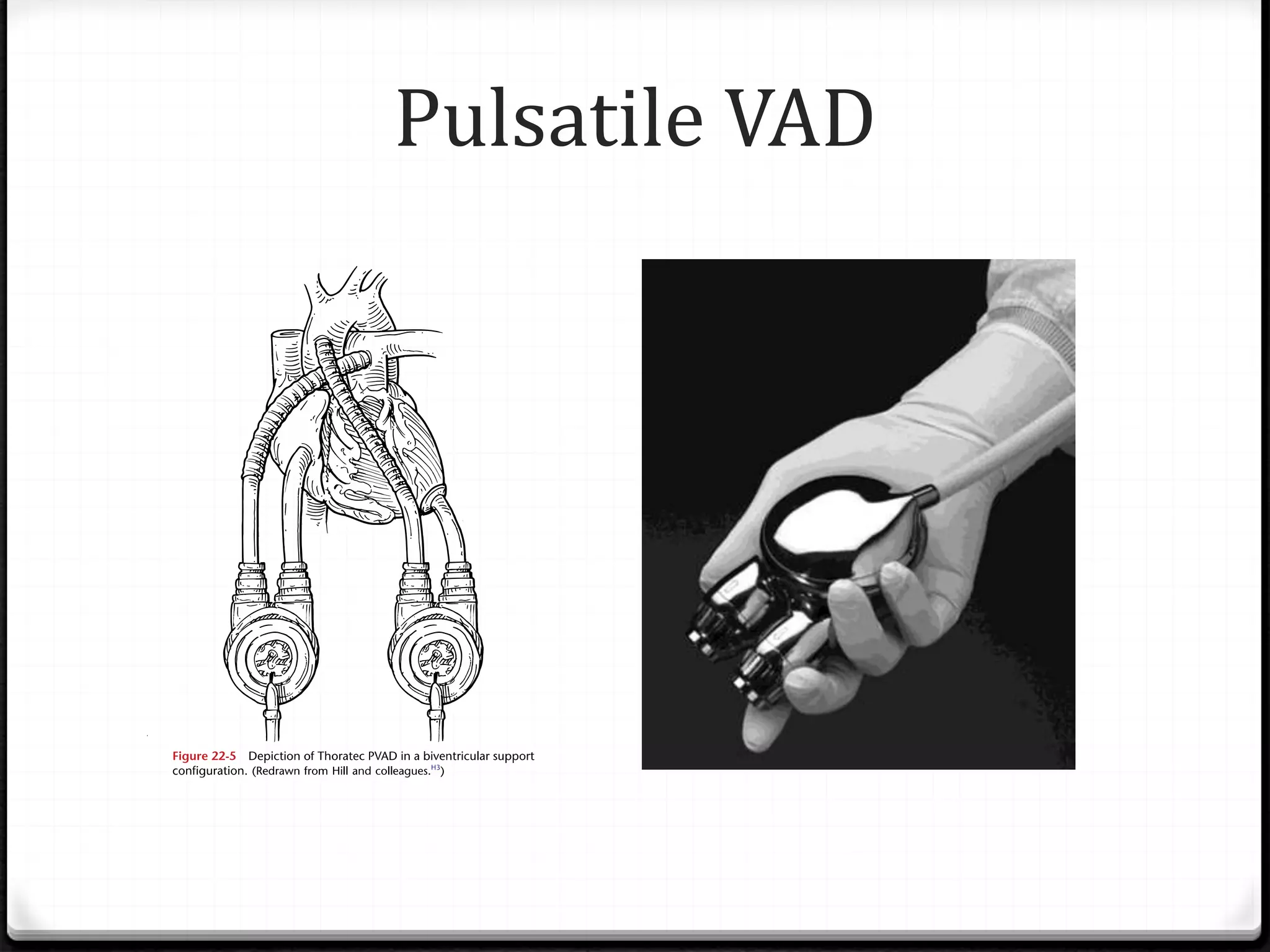
Ventricular assist devices (VADs) are mechanical pumps that help the failing heart pump blood. There are two main types - pulsatile VADs that pump blood in pulses and continuous flow VADs that pump blood continuously. VADs are used as a bridge to heart transplant, to help the heart recover, or as destination therapy for those not eligible for transplant. They are implanted using cardiopulmonary bypass to connect the inflow cannula in the left ventricle to the outflow cannula in the aorta. VADs improve heart function and quality of life for patients with heart failure.