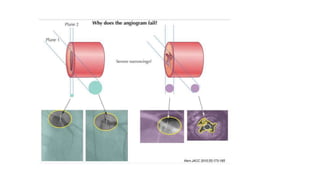
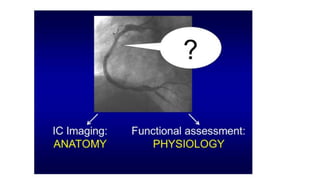
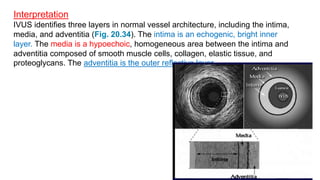
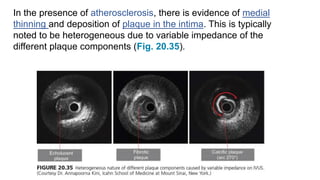
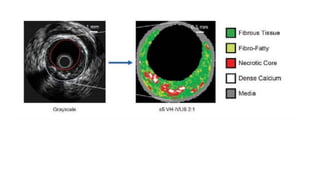
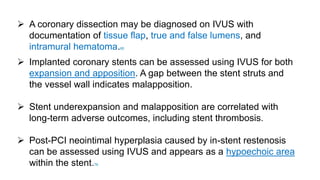
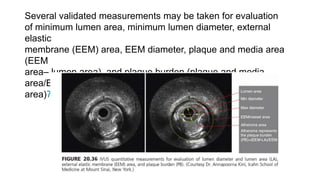
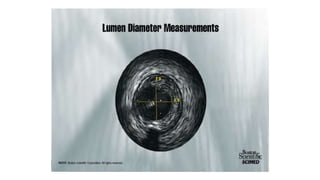
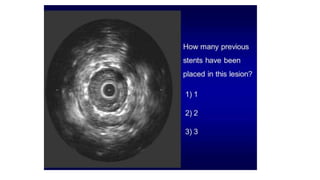
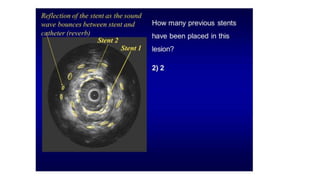
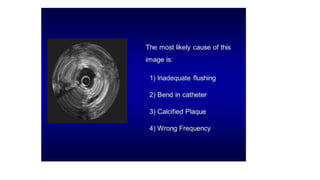
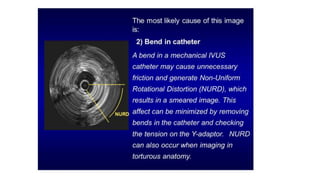
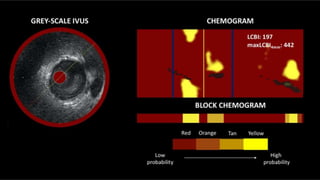
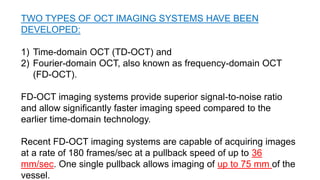
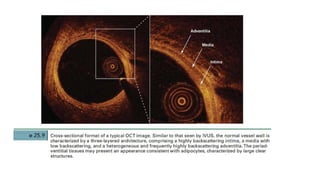
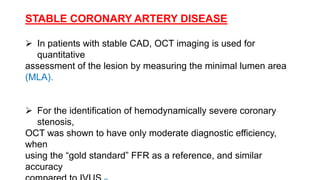
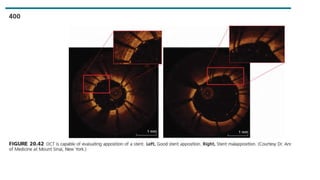
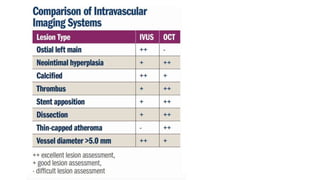
This document discusses intravascular ultrasound (IVUS) and optical coherence tomography (OCT) for assessing coronary artery disease.
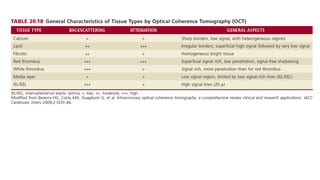
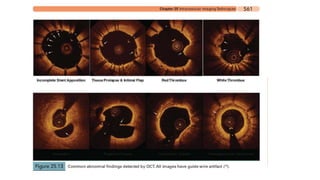
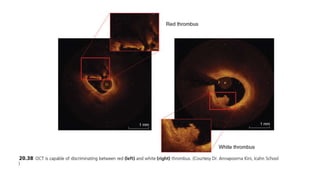
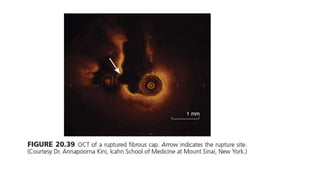
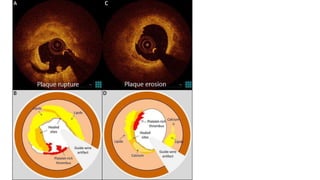
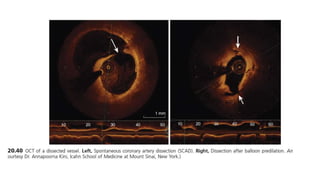
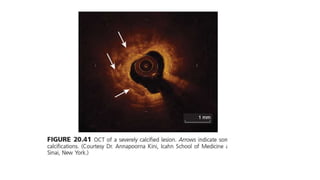
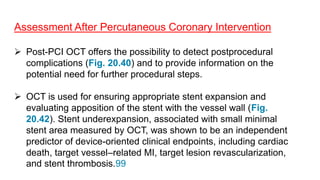
IVUS uses sound waves to image vessel walls with good penetration but lower resolution compared to OCT. Virtual histology IVUS can characterize plaque morphology. Studies show IVUS guidance for percutaneous coronary intervention reduces major adverse cardiac events. OCT uses near-infrared light for very high resolution imaging of plaque, thrombus, dissections and stent apposition. It guides lesion preparation and detects post-PCI complications. Both modalities provide detailed vessel and plaque assessment to optimize revascularization.