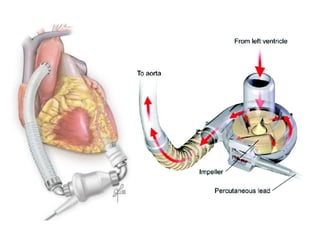
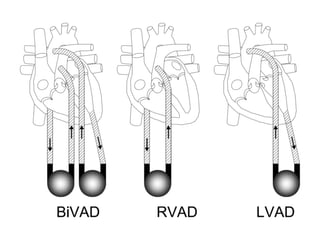
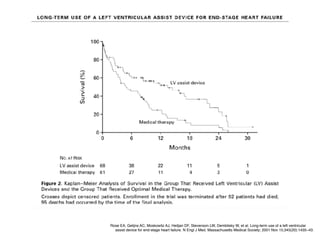
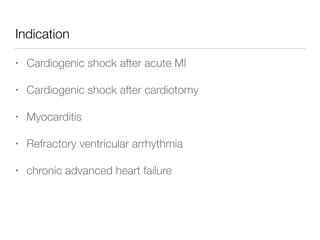
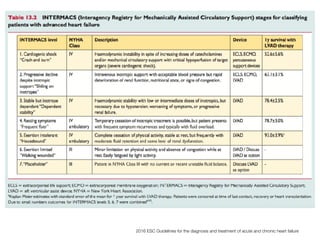
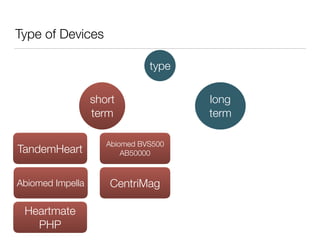
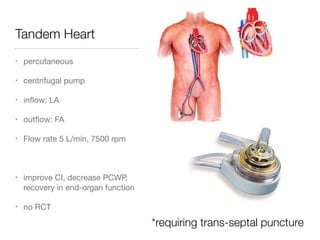
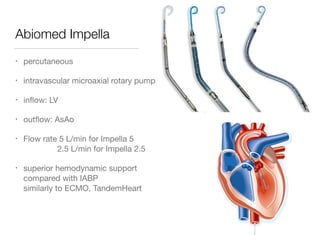
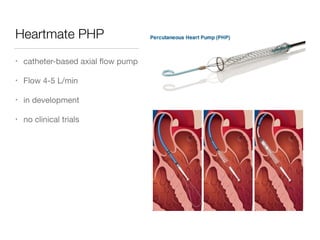
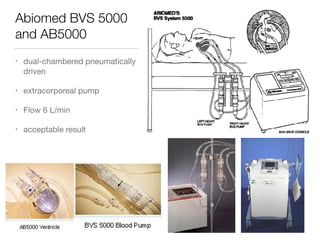
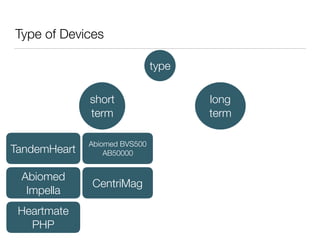
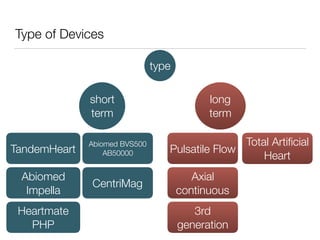
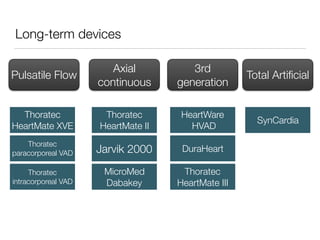
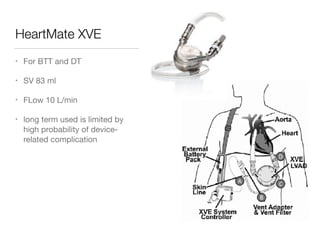
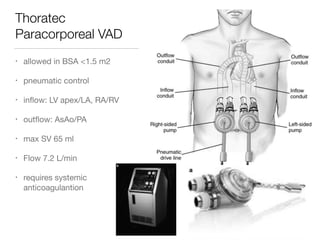
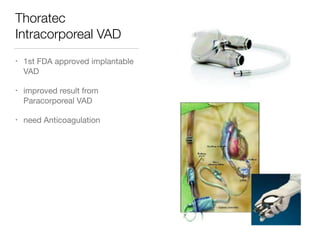
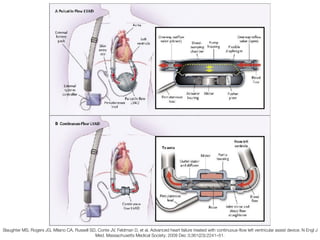
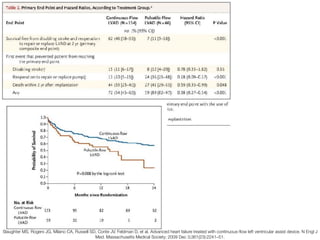
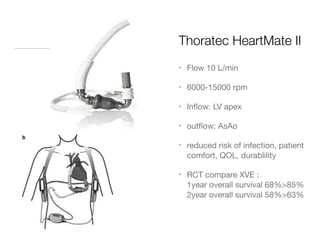
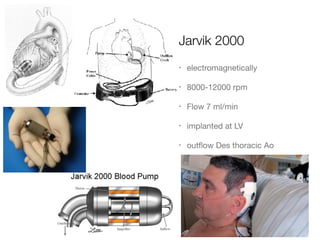
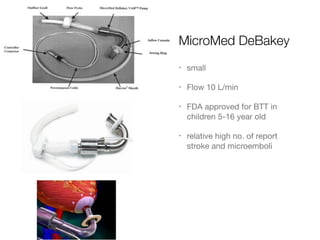
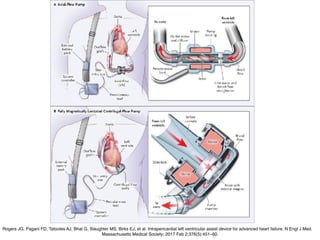
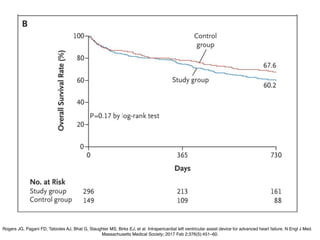
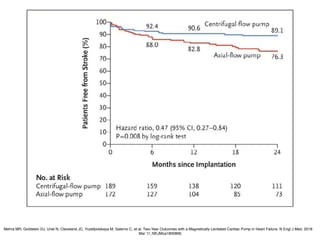
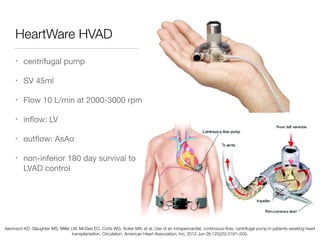
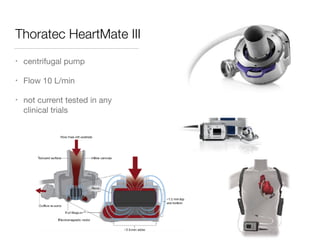
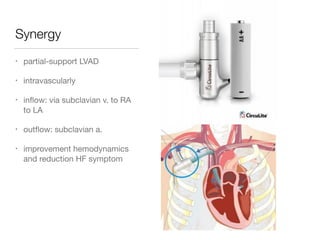
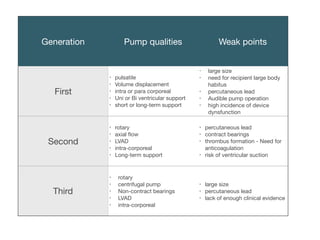
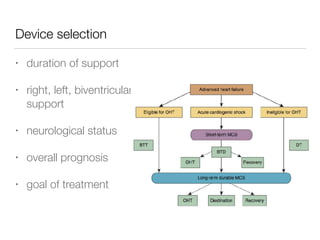
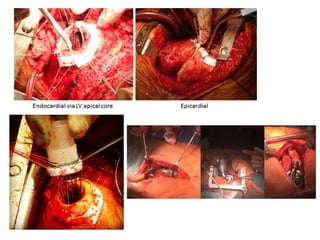
Ventricular assist devices (VADs) are mechanical pumps that help the failing heart pump blood. They can be used as a bridge to transplantation, destination therapy for those not eligible for transplant, or as a bridge to recovery or decision. VADs range from short-term percutaneous devices to long-term implantable devices. Long-term devices include pulsatile flow, axial continuous flow, and total artificial hearts. Selection depends on duration of support needed, whether right or left ventricular support is required, and the patient's prognosis and treatment goals. Implantation requires open-heart surgery and postoperative management focuses on prevention of complications like bleeding, infection, and thromboembolism.