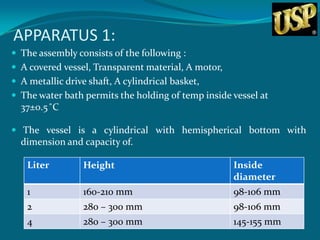
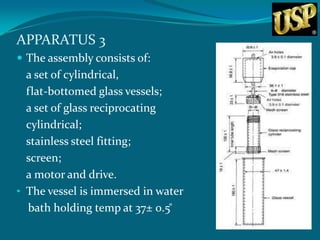
This document summarizes USP dissolution testing apparatus and procedures. It describes 7 different apparatus for testing dissolution of solid oral dosage forms including baskets, paddles, cylinders and flow-through cells. Key steps are outlined for each apparatus, including preparation of the dosage form sample, equilibrating the dissolution medium, operating conditions, sampling time points and procedures. Temperatures and rotations speeds are specified. The goal is to perform dissolution testing under standardized and reproducible conditions to evaluate drug release characteristics.