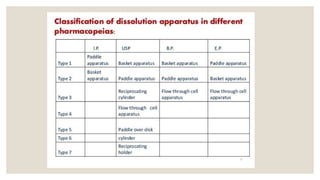
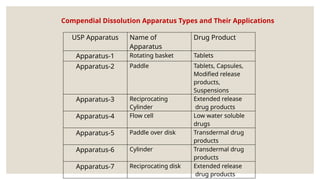
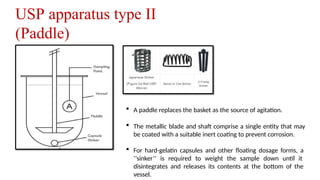
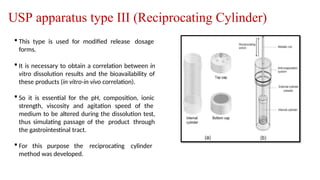
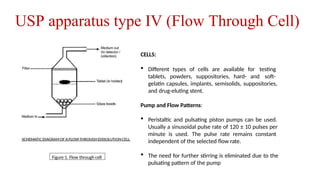
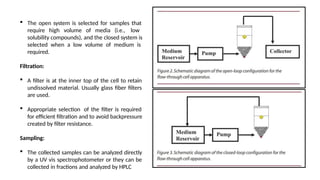
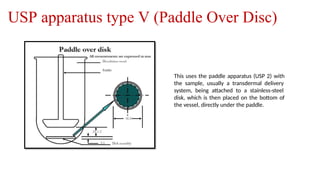
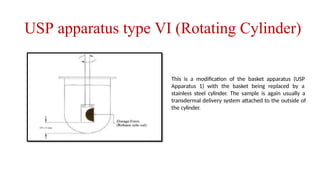
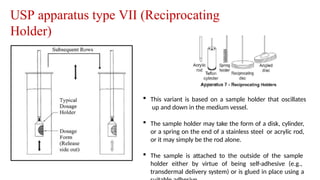
The document discusses the process of dissolution, which is crucial for determining the bioavailability and quality of drug products. It outlines various compendial methods and apparatus for conducting dissolution testing, including types like rotating basket, paddle, reciprocating cylinder, and flow-through cell, along with their respective applications and issues. Additionally, it highlights factors influencing test design and the significance of in vitro dissolution testing for regulatory approval.