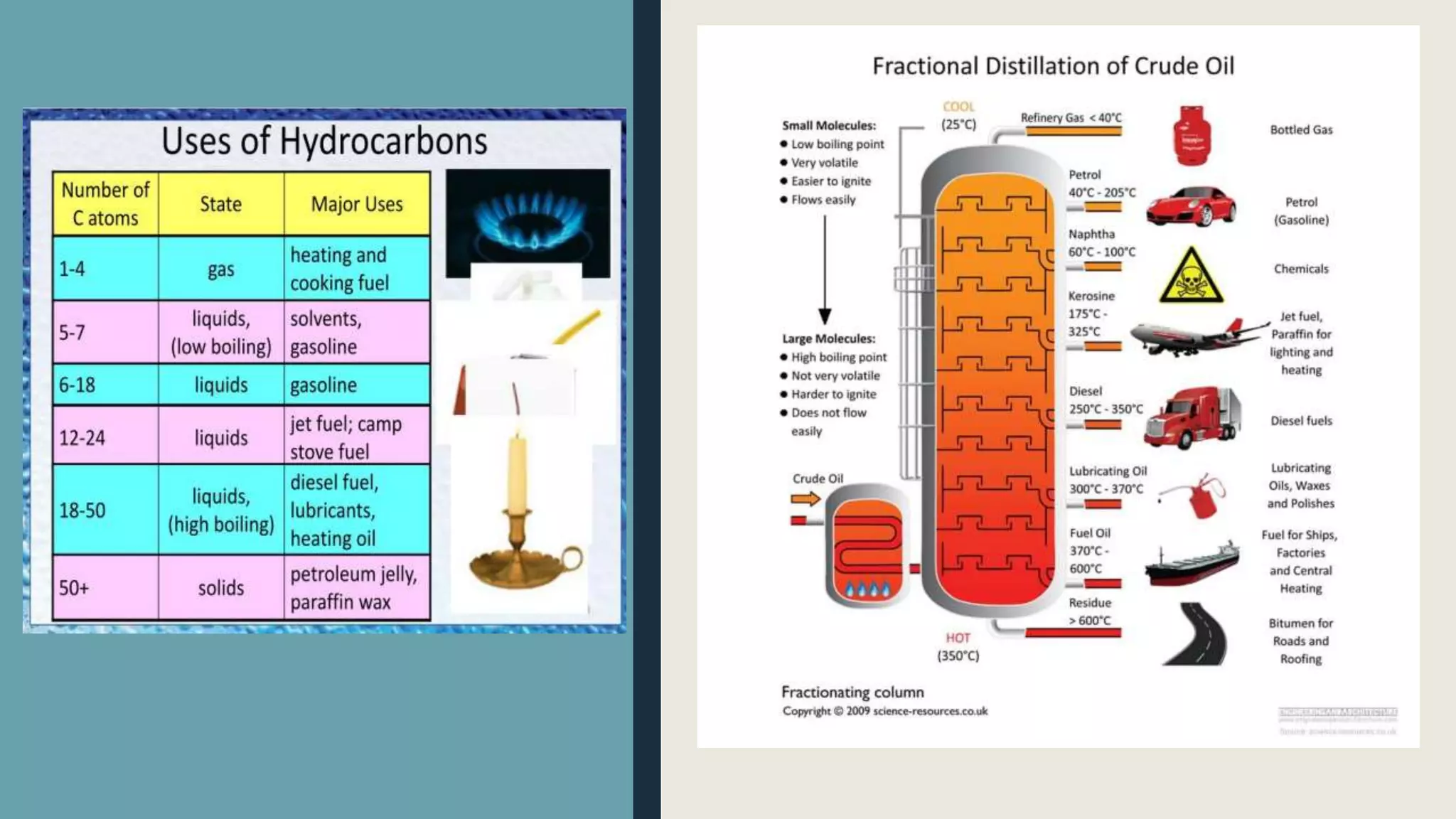
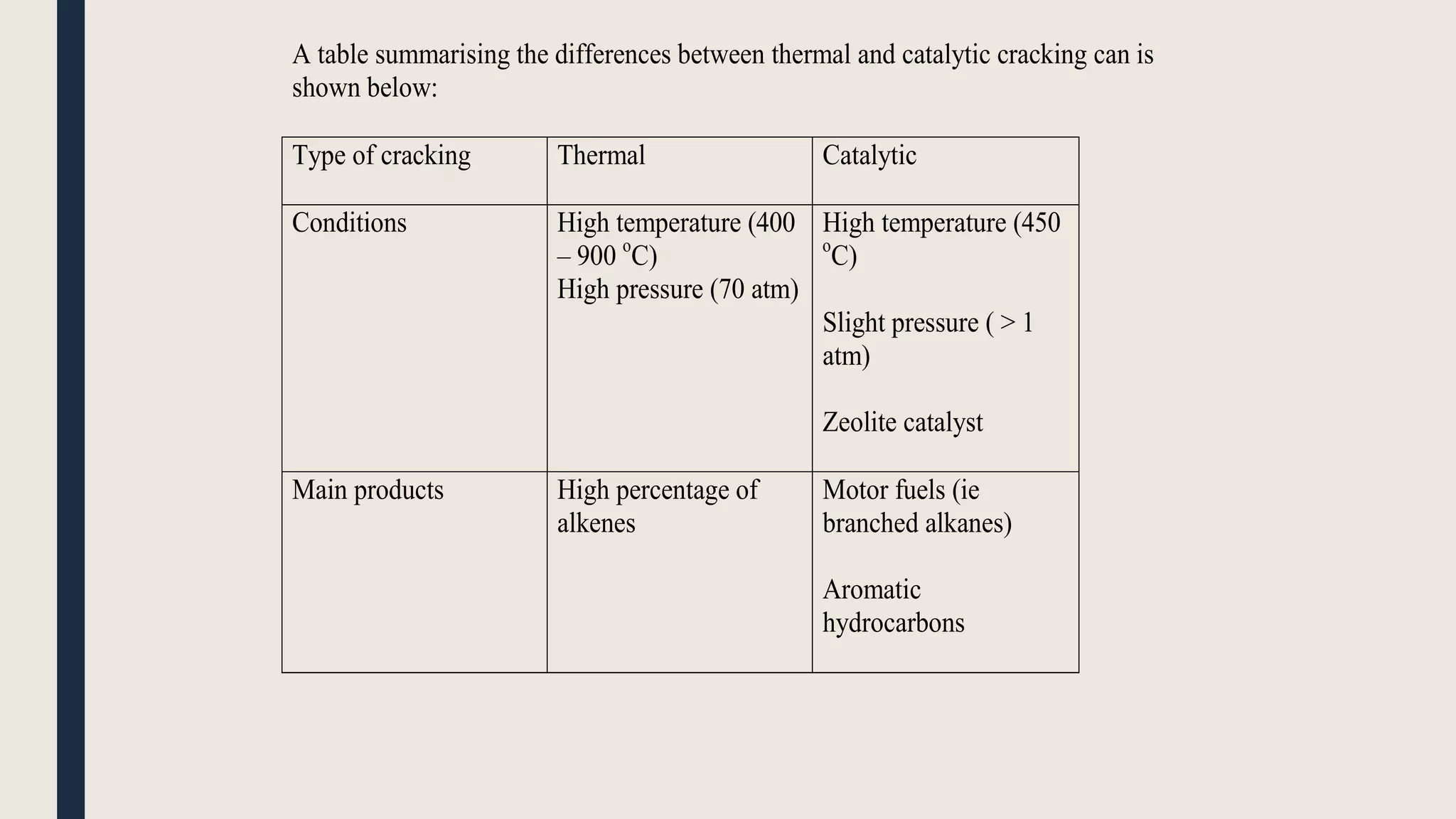
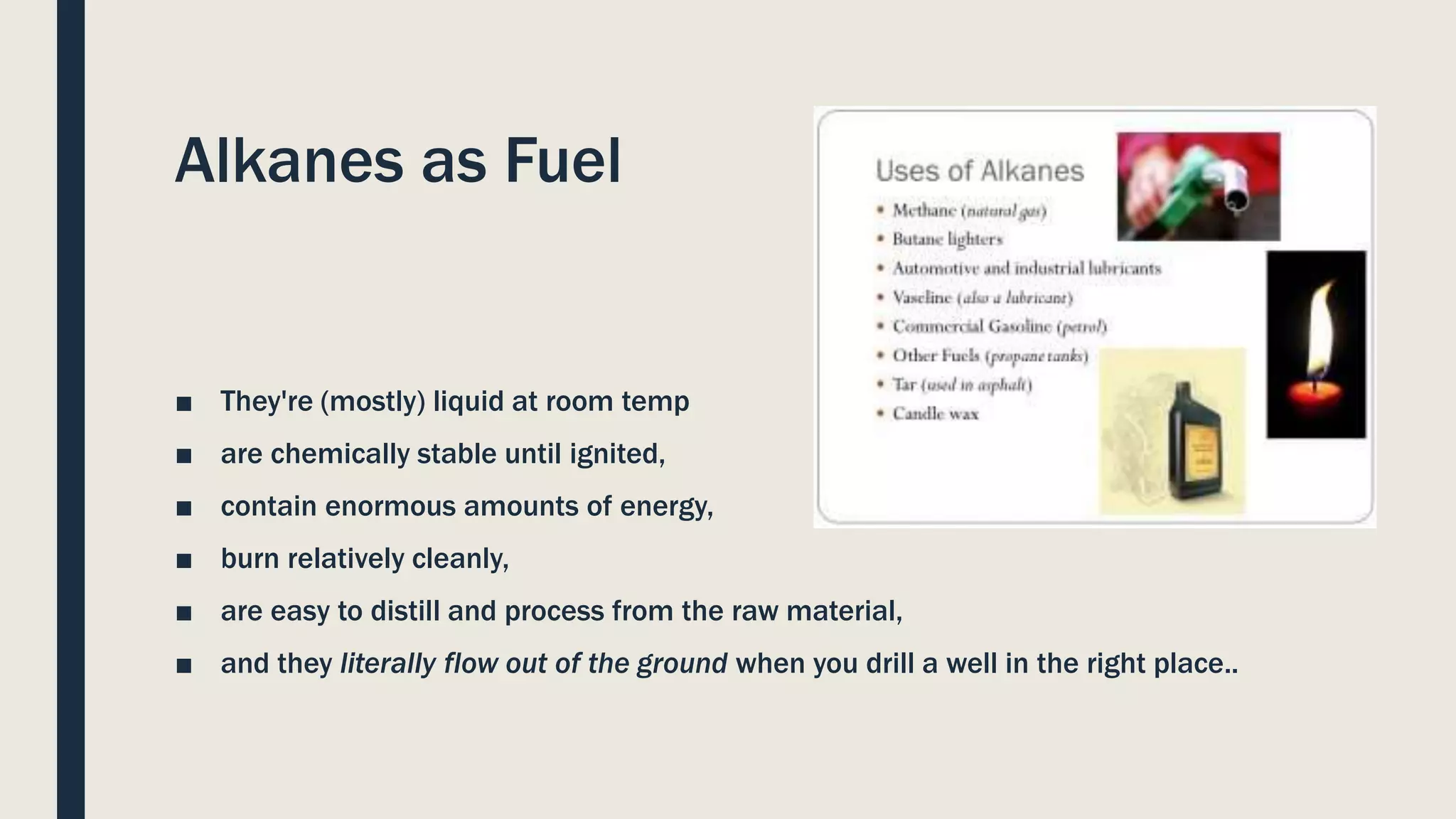
Hydrocarbons can be separated from crude oil using fractional distillation based on their different boiling points. Long chain hydrocarbons can be cracked into shorter chains through thermal or catalytic cracking. Thermal cracking uses high temperature and pressure while catalytic cracking uses lower temperature, pressure and a zeolite catalyst. Alkanes have various uses as fuels, solvents, and in biogas production where bacteria convert organic waste into methane gas. Alkenes are used to produce important polymers like polyethylene, polypropylene, and PVC.