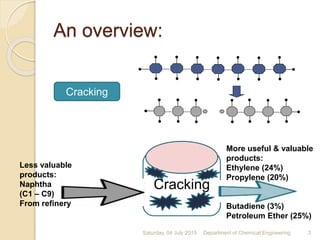
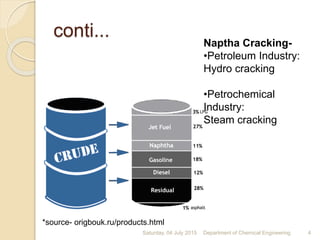
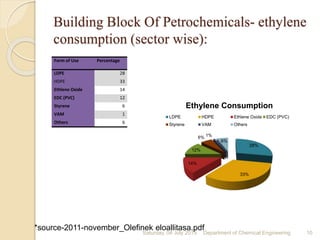
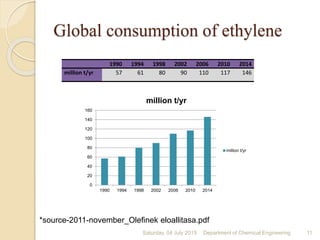
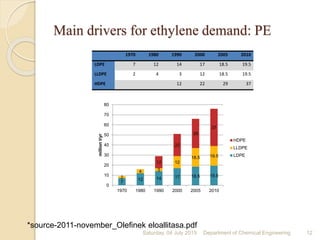
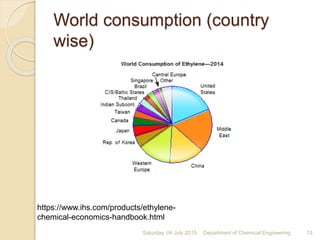
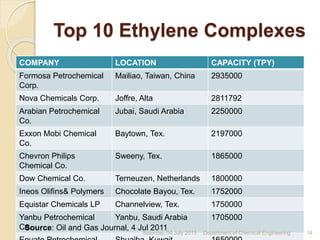
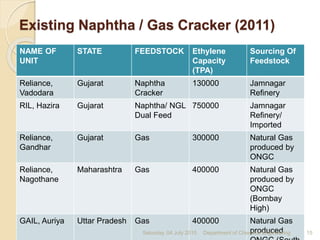
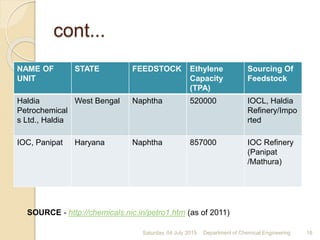
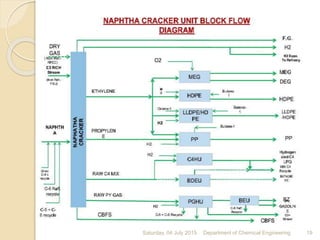
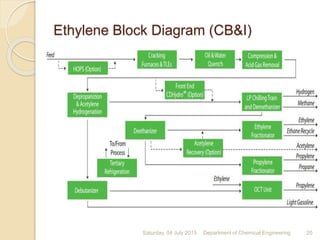
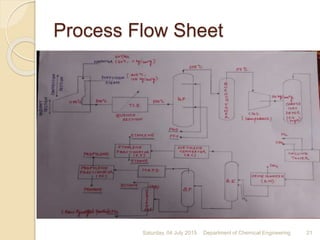
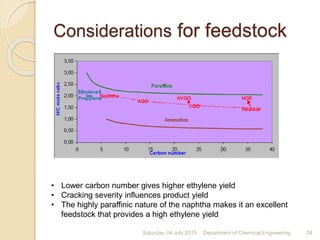
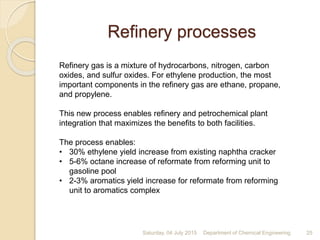
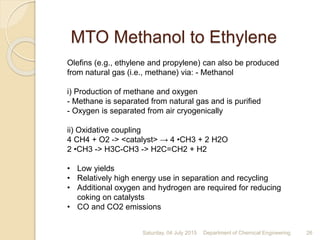
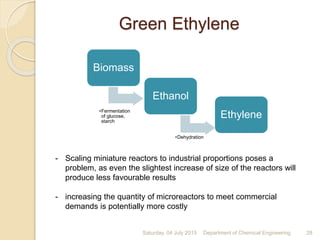
The document summarizes ethylene production via naphtha cracking. It provides an overview of the history and development of naphtha cracking and steam cracking processes. It also discusses global ethylene consumption trends, production processes like steam cracking, MTO, and green routes. The key drivers of increasing ethylene demand are highlighted as polyethylene production. Major existing naphtha crackers in India and their capacities are listed. Process design considerations for ethylene plants emphasize safety, energy efficiency, and reliability.