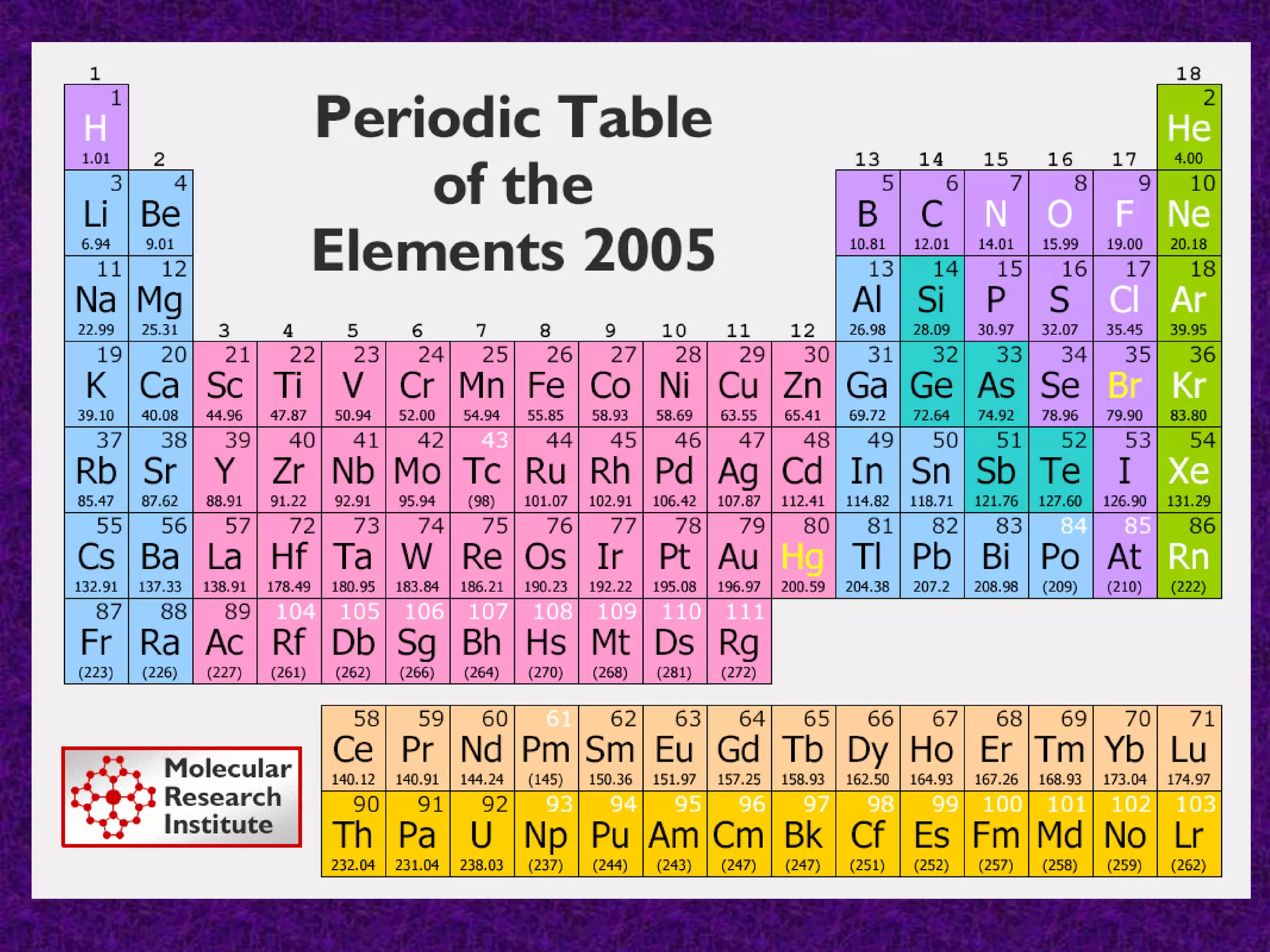
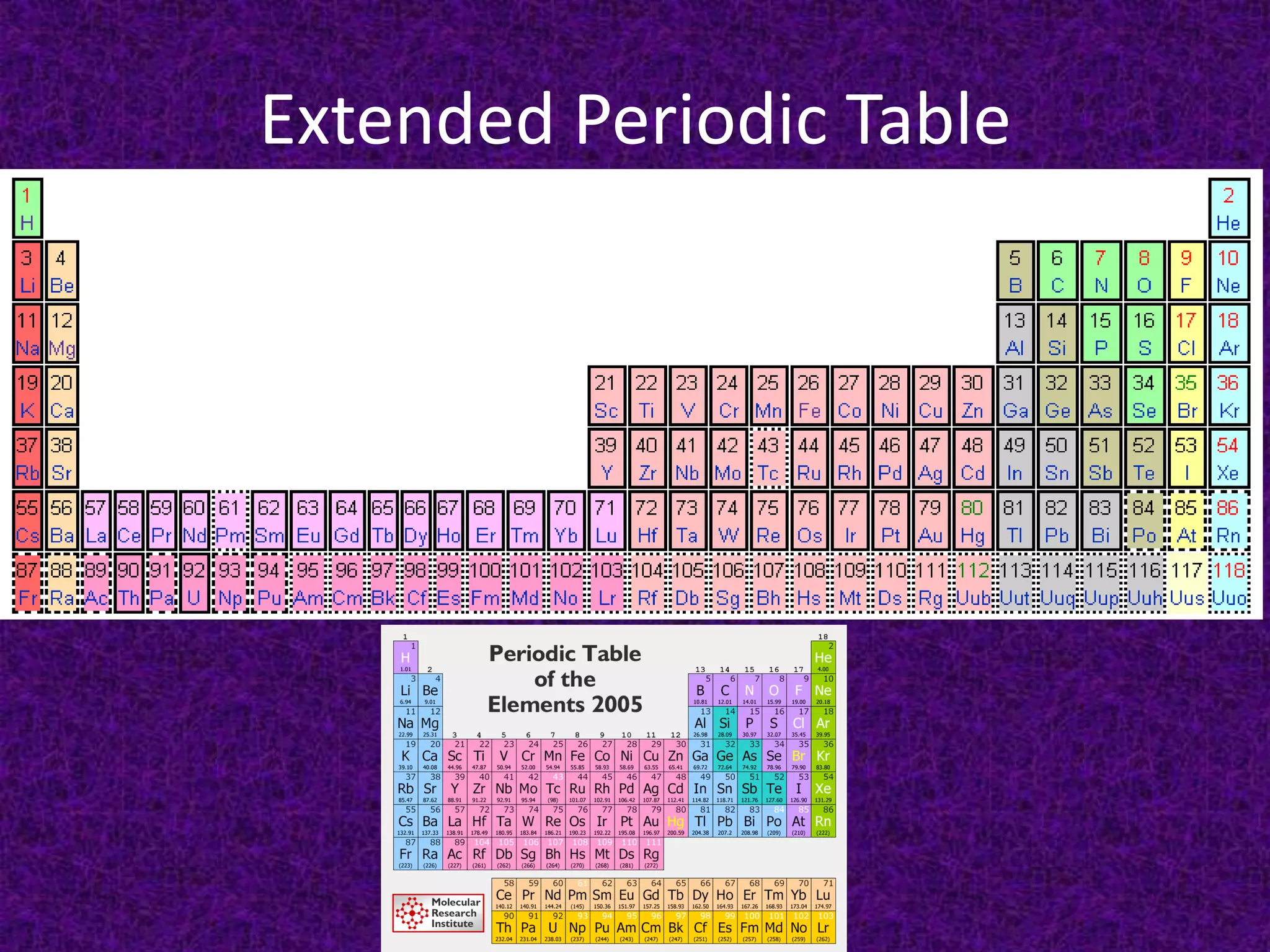
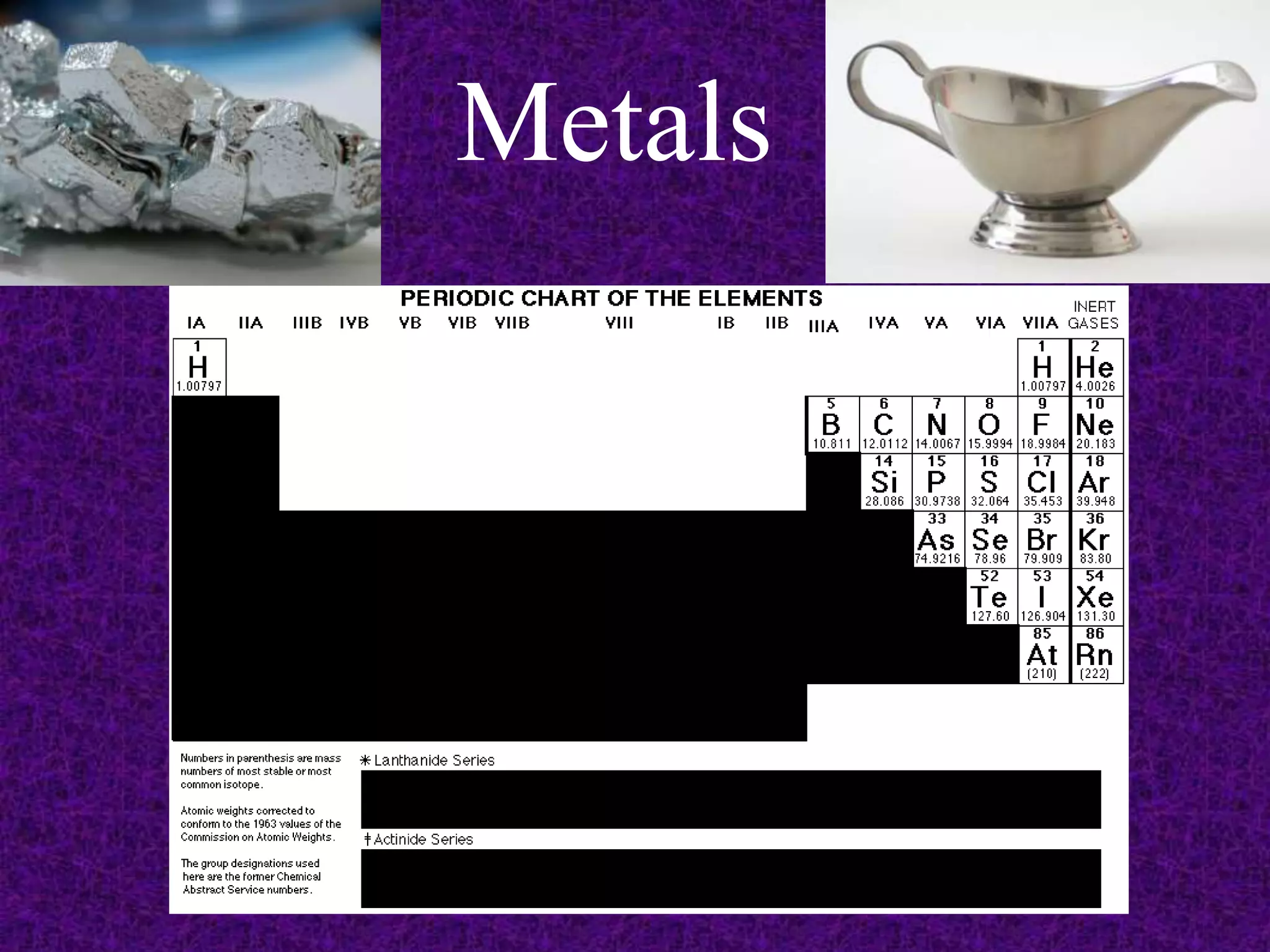
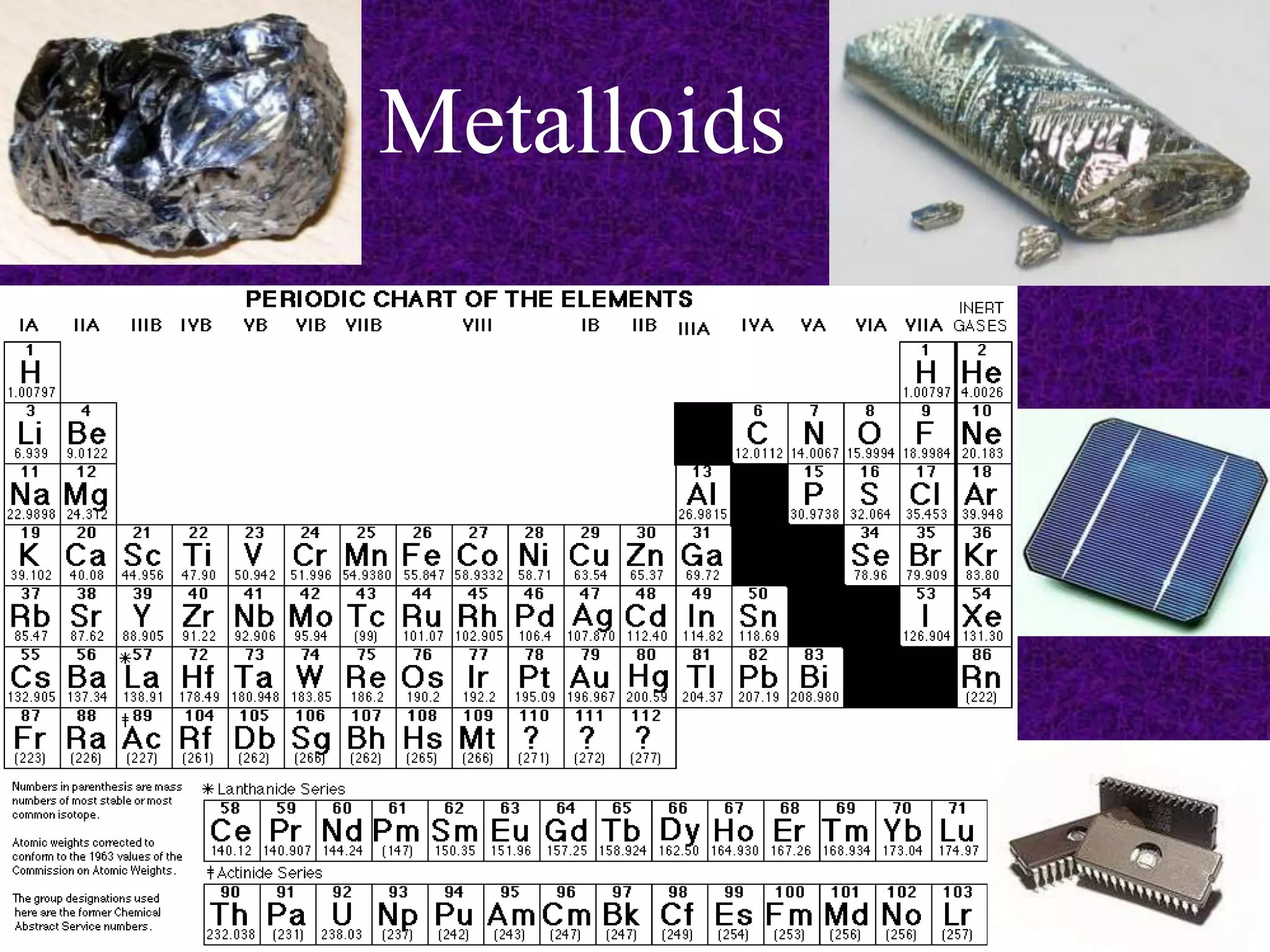
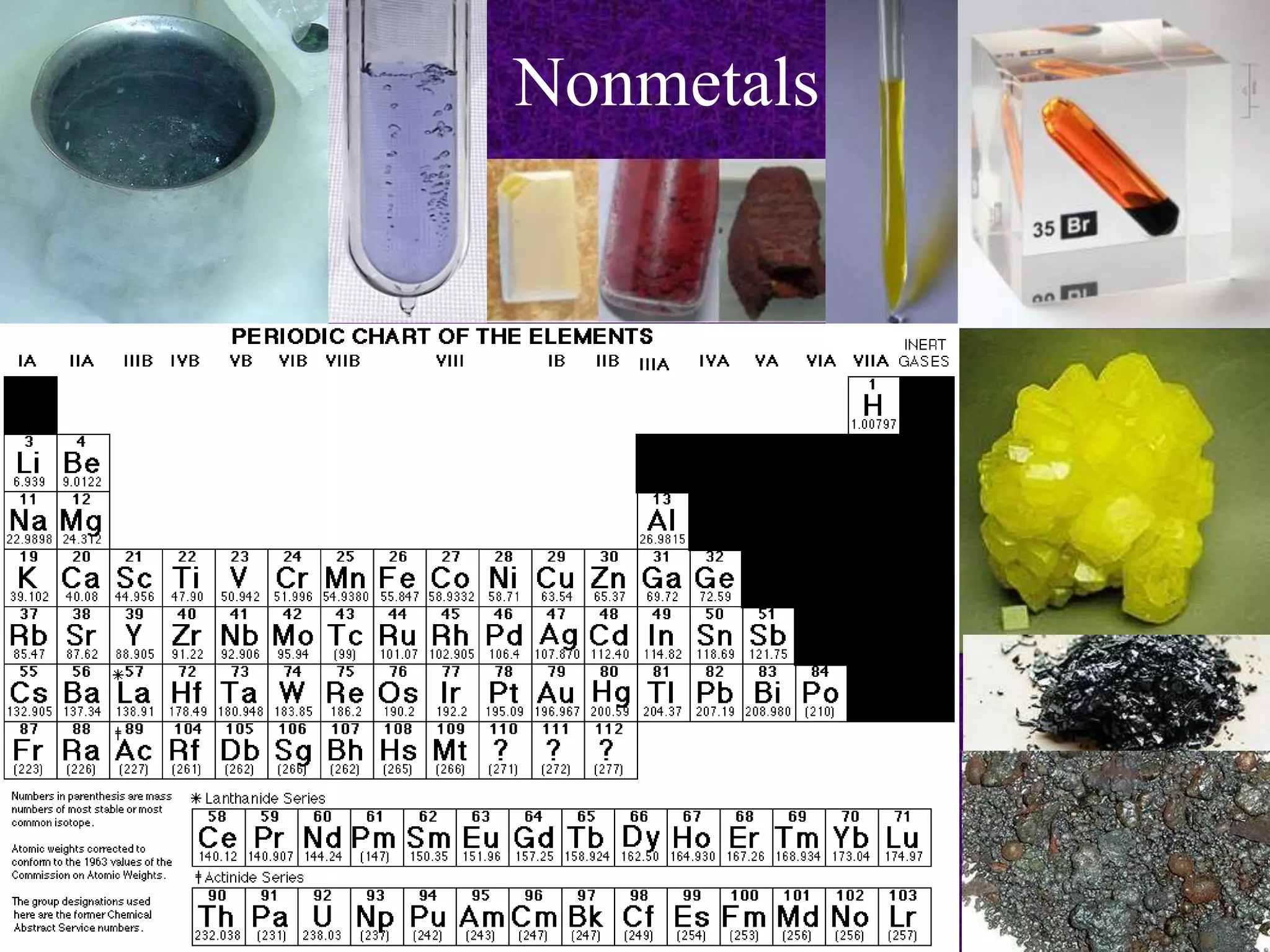
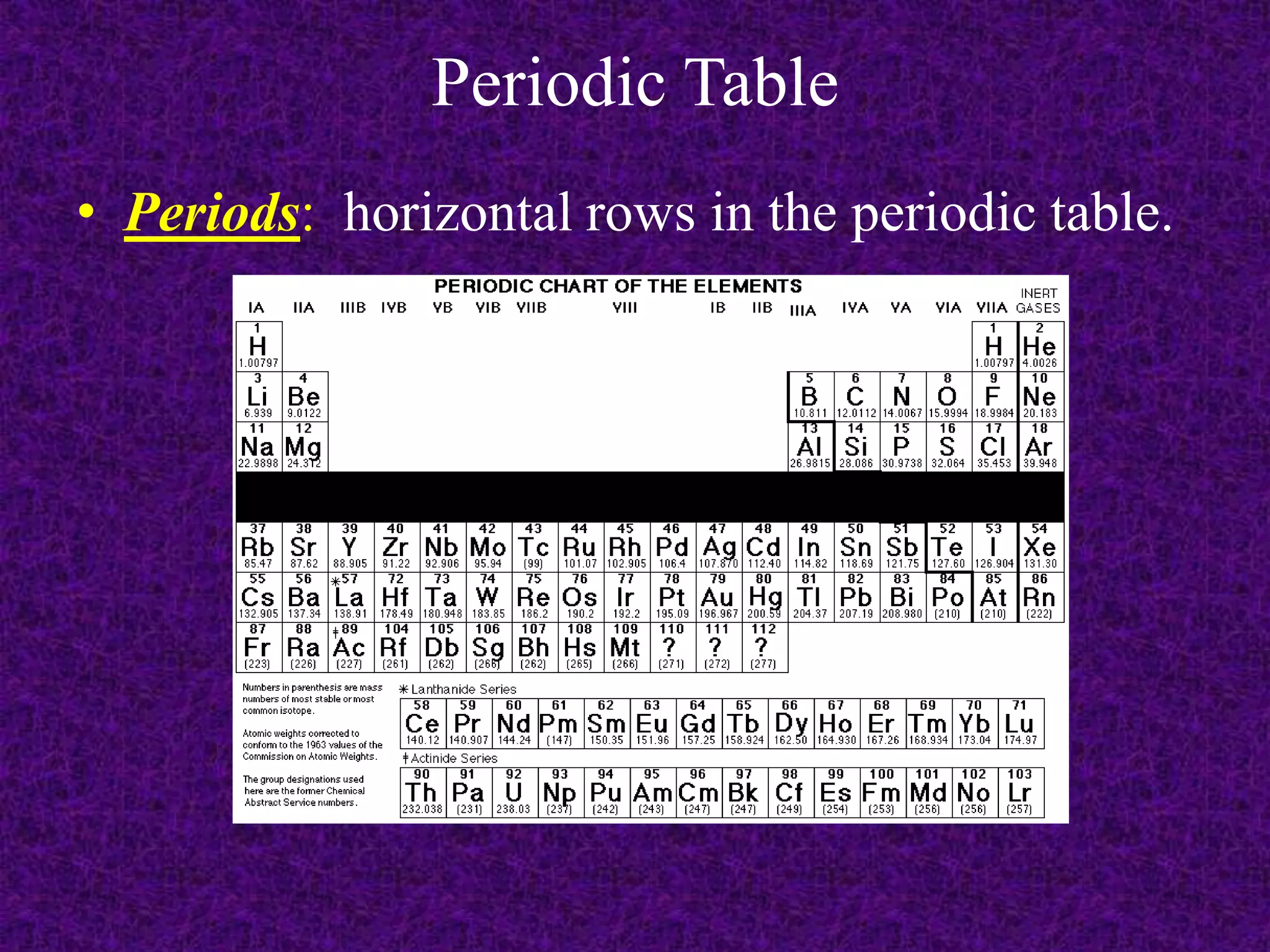
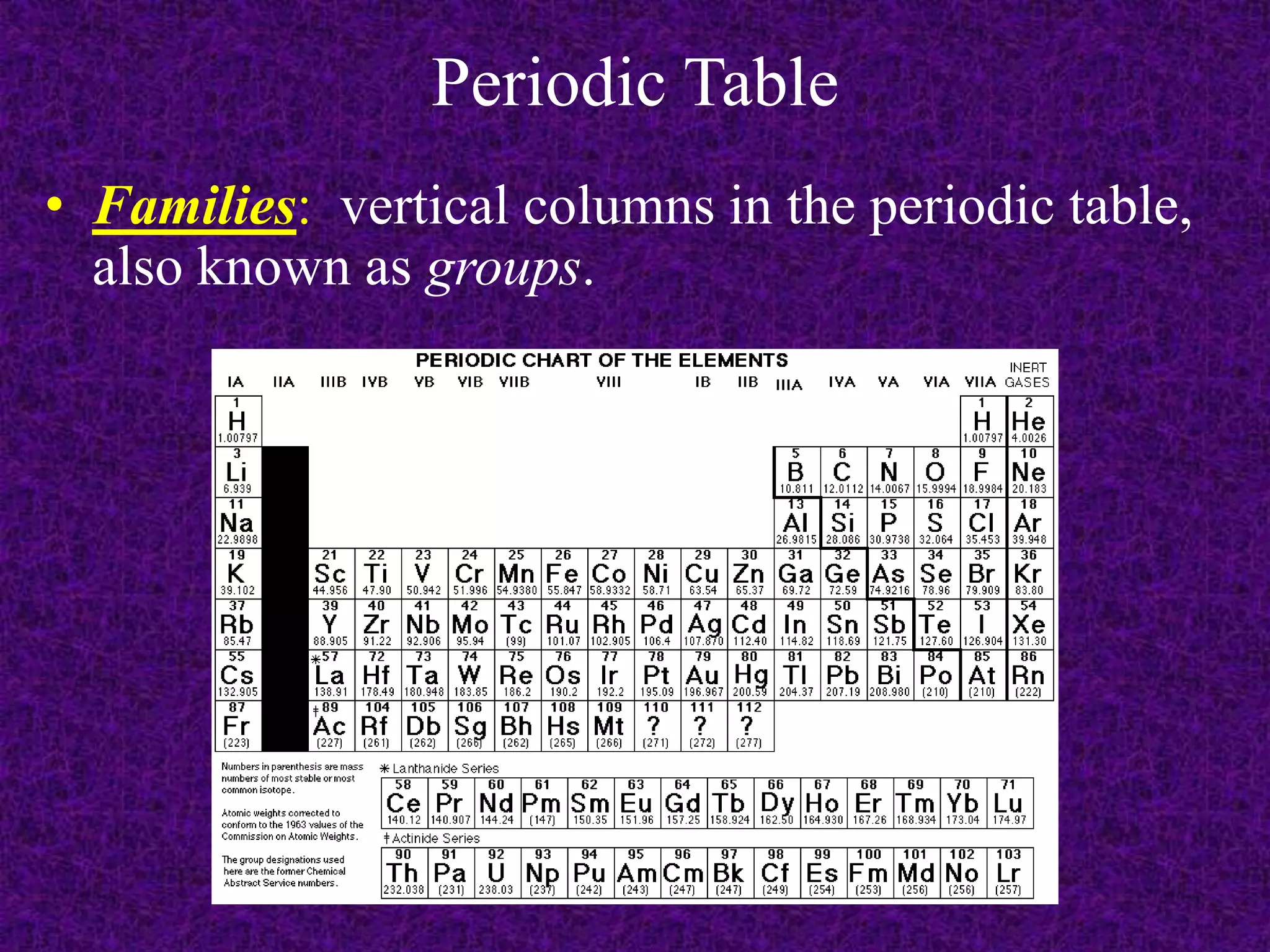
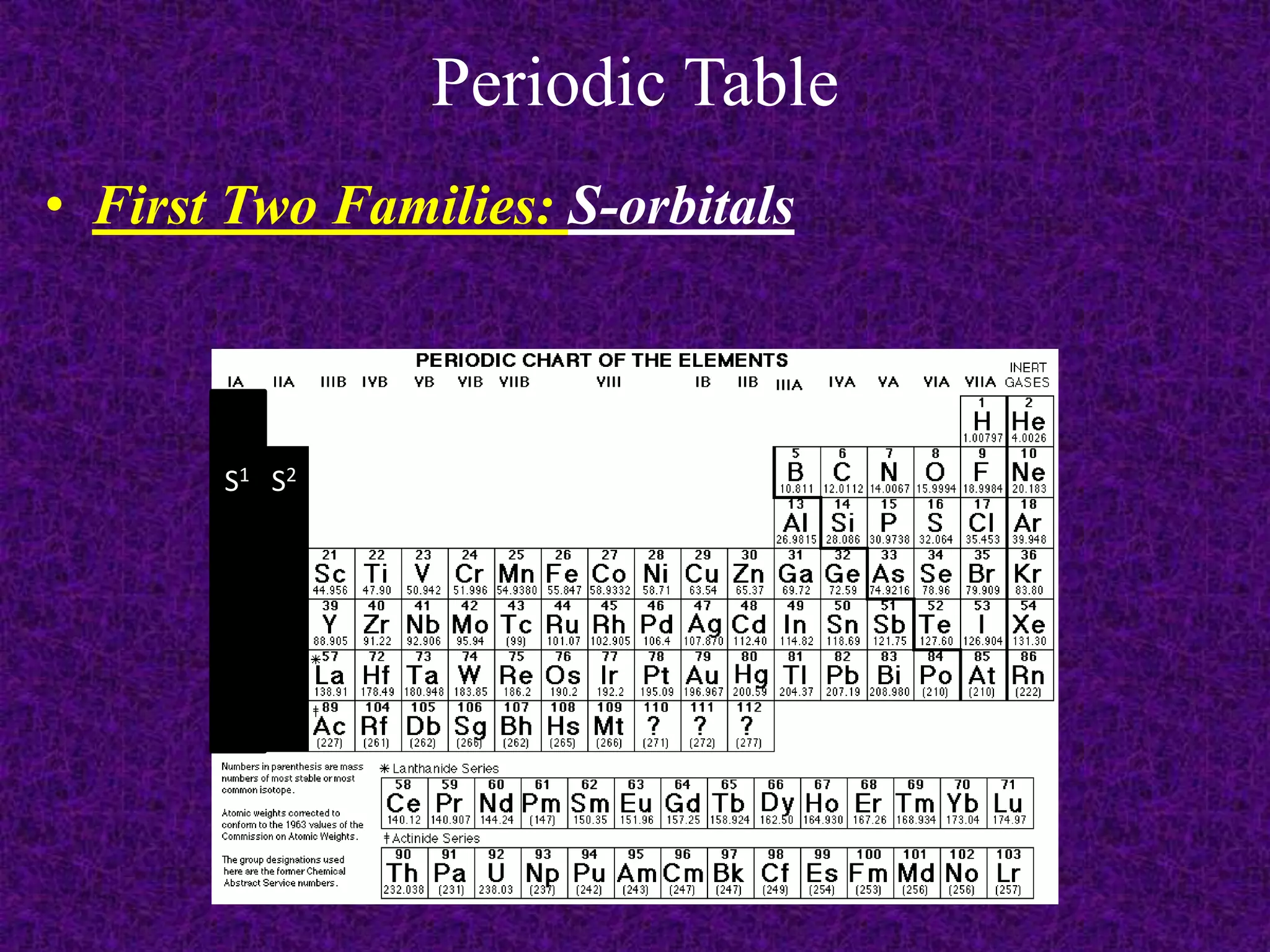
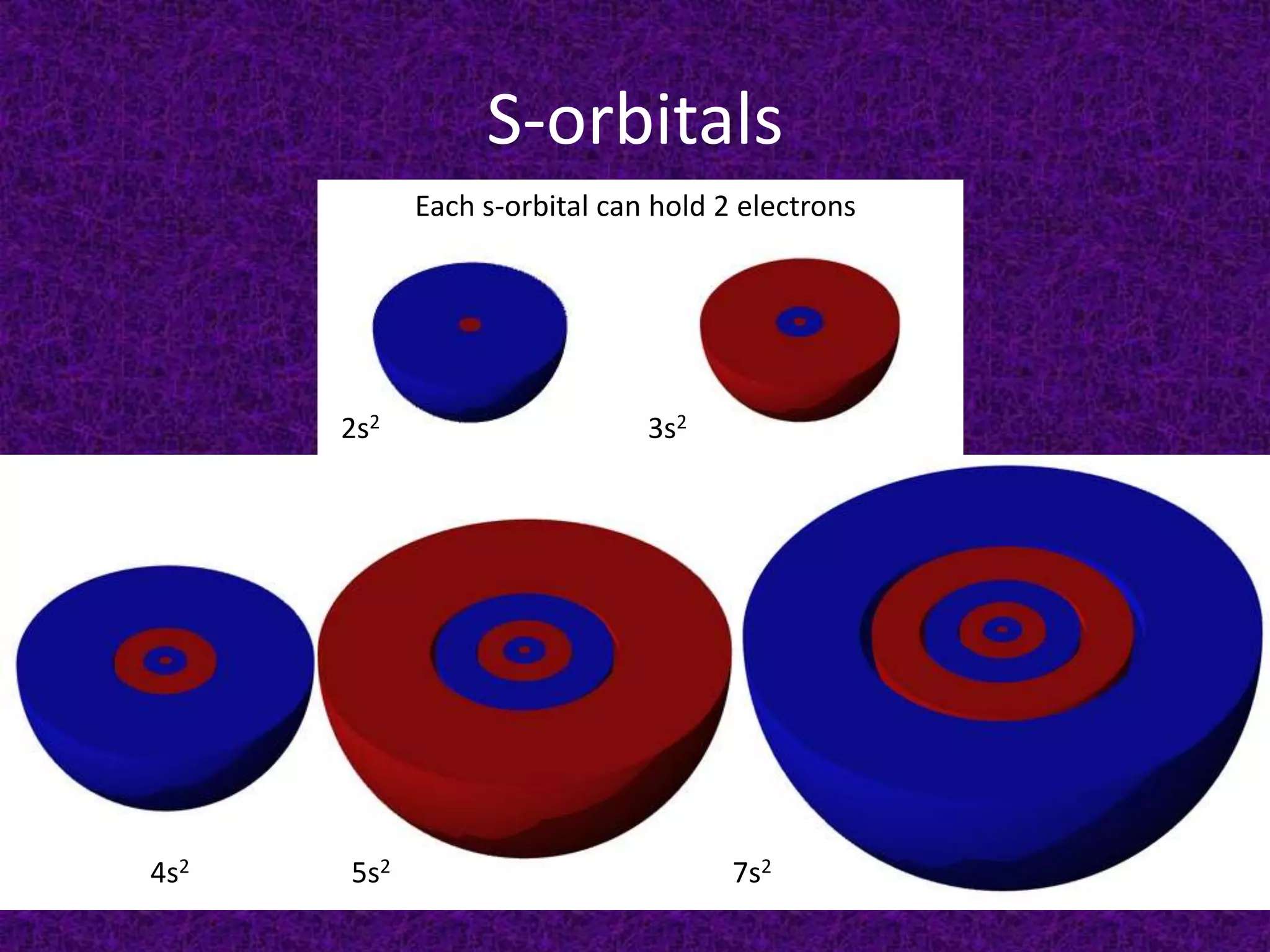
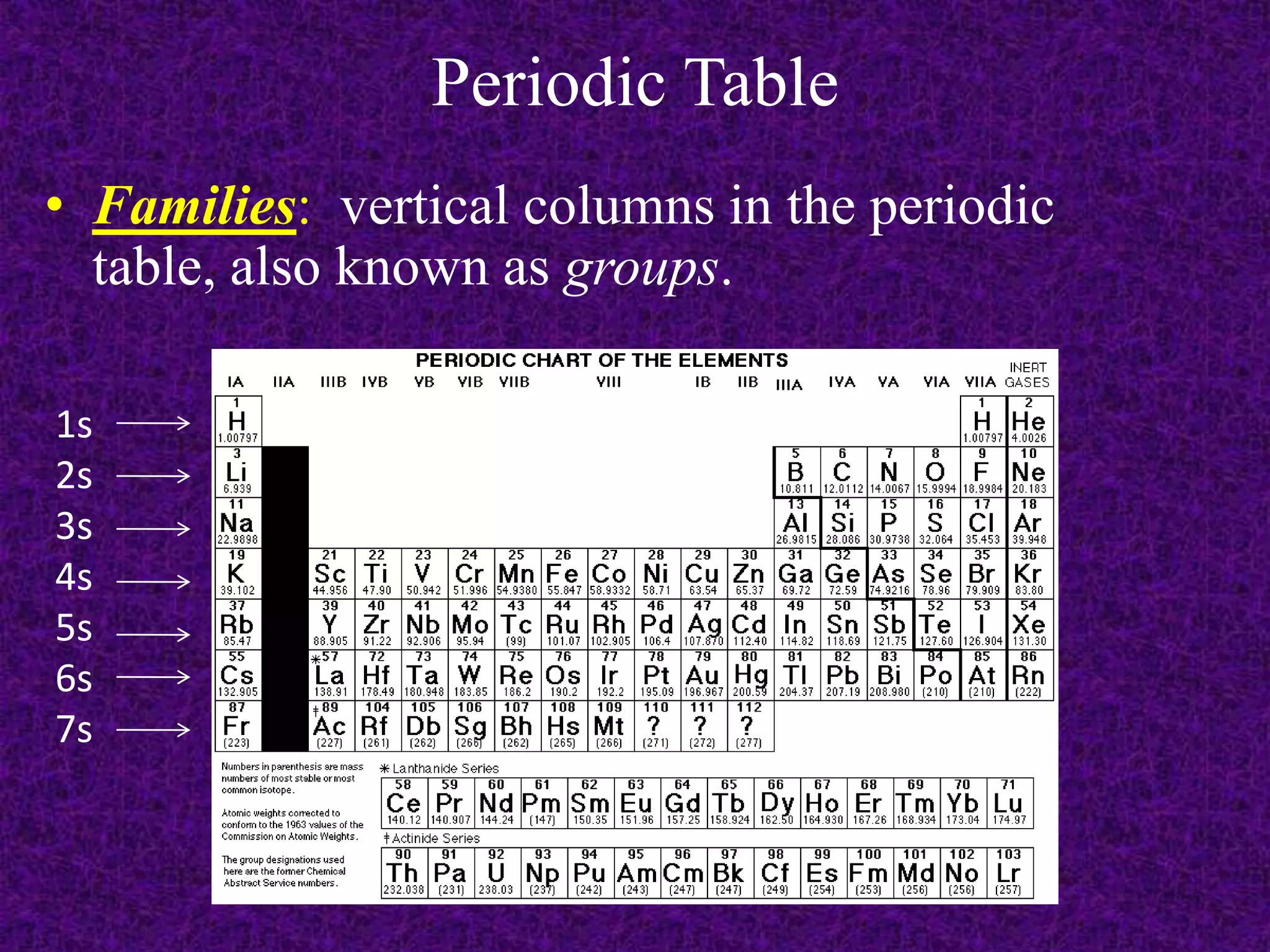
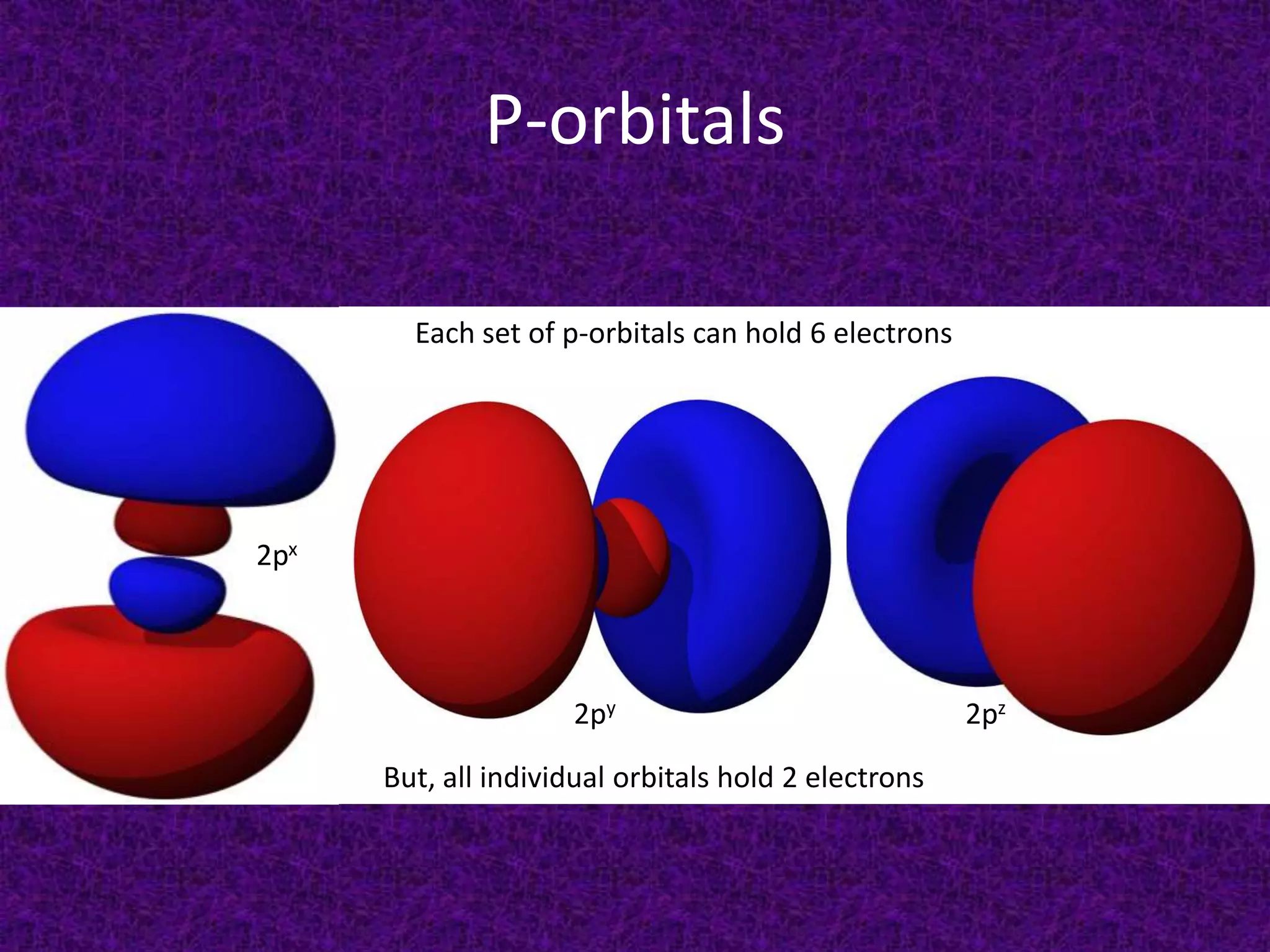
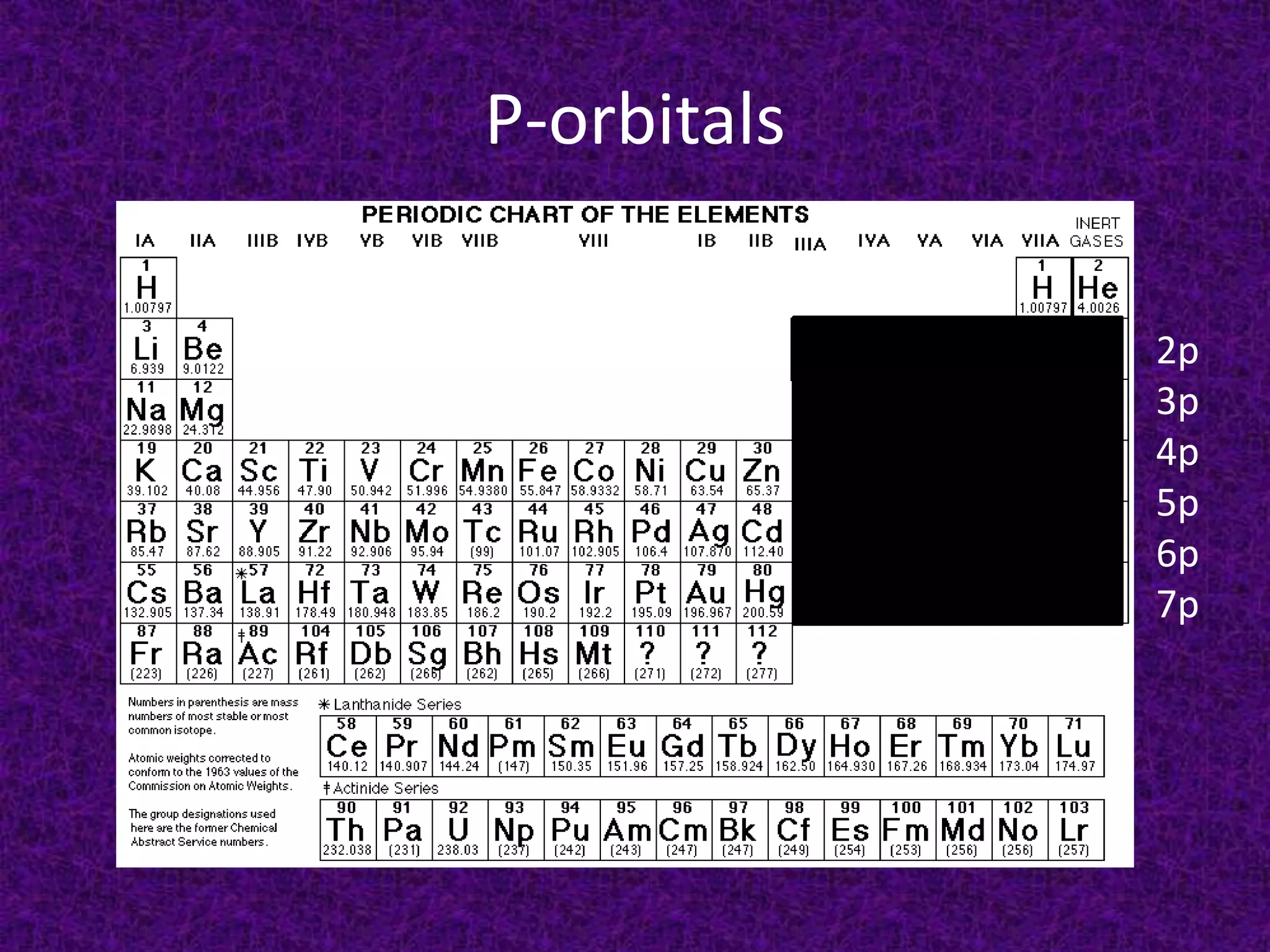
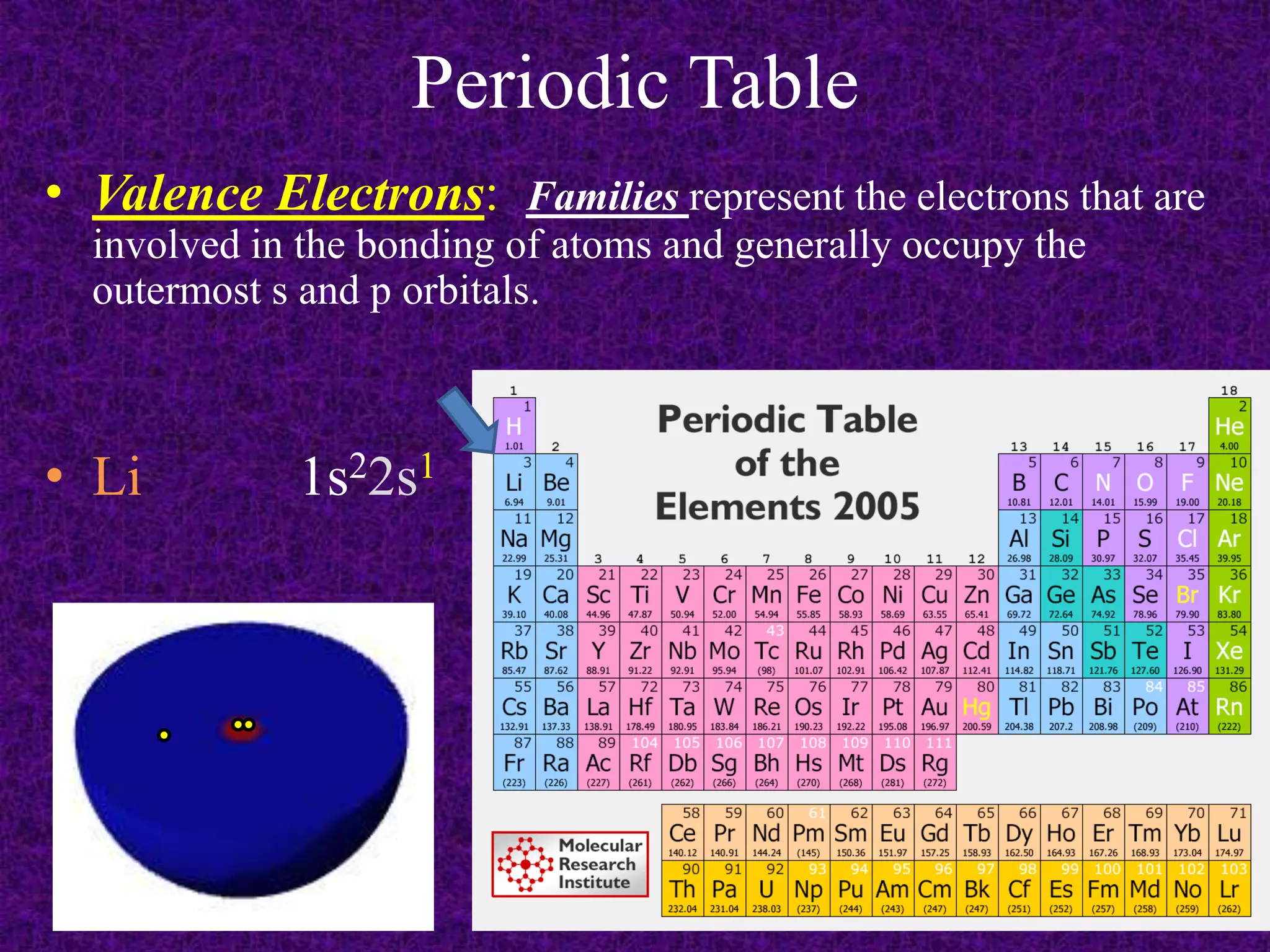
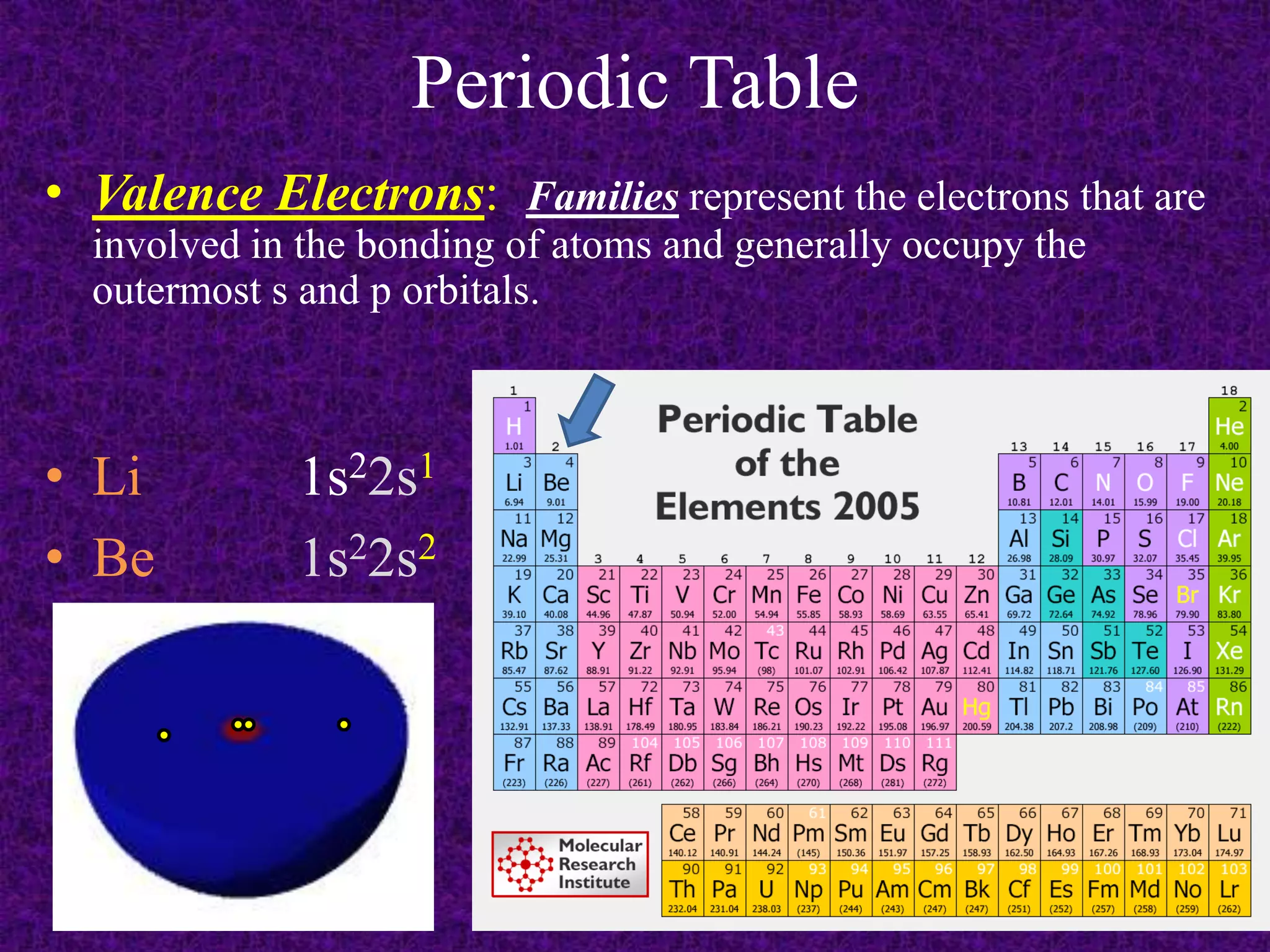
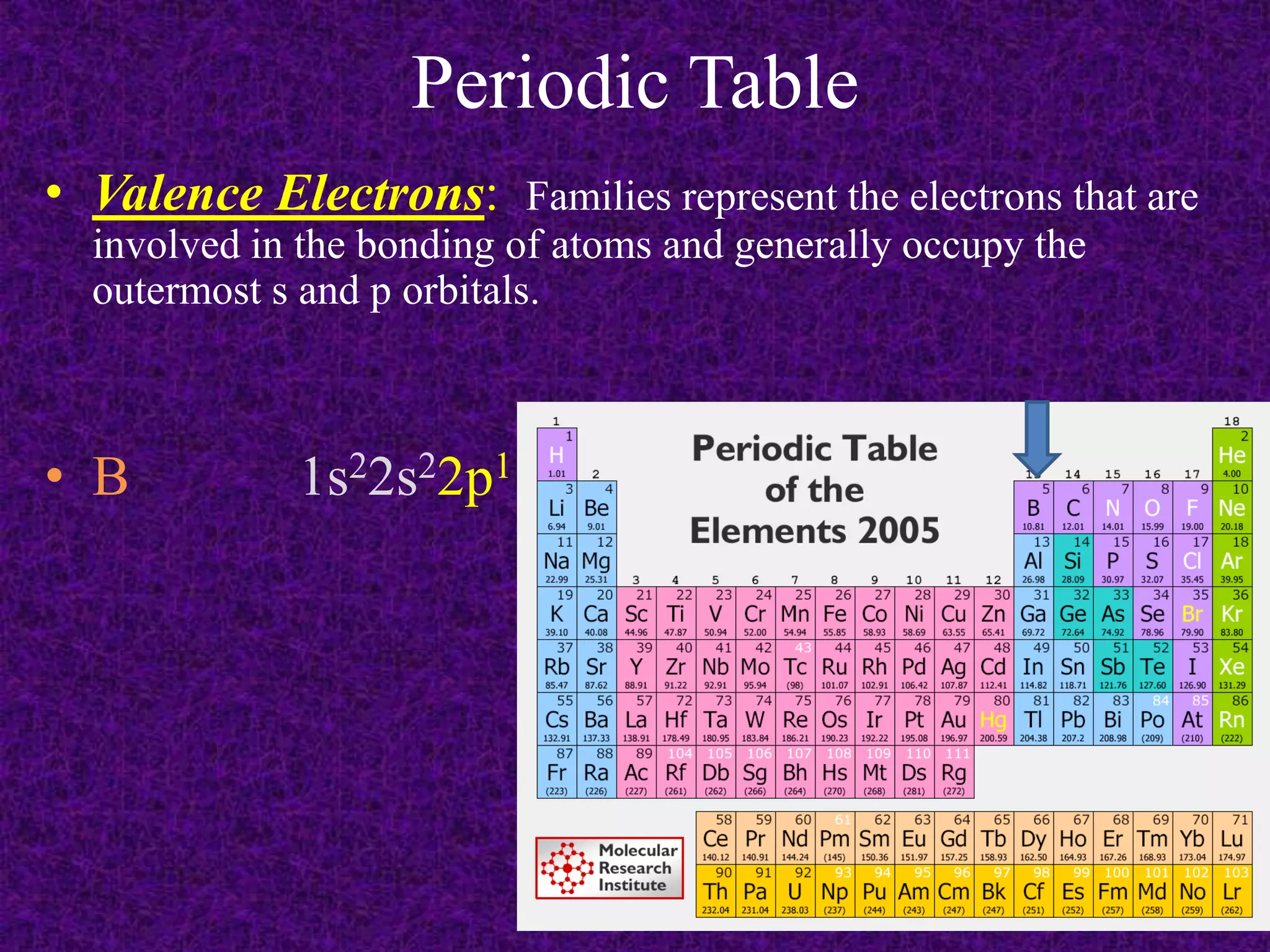
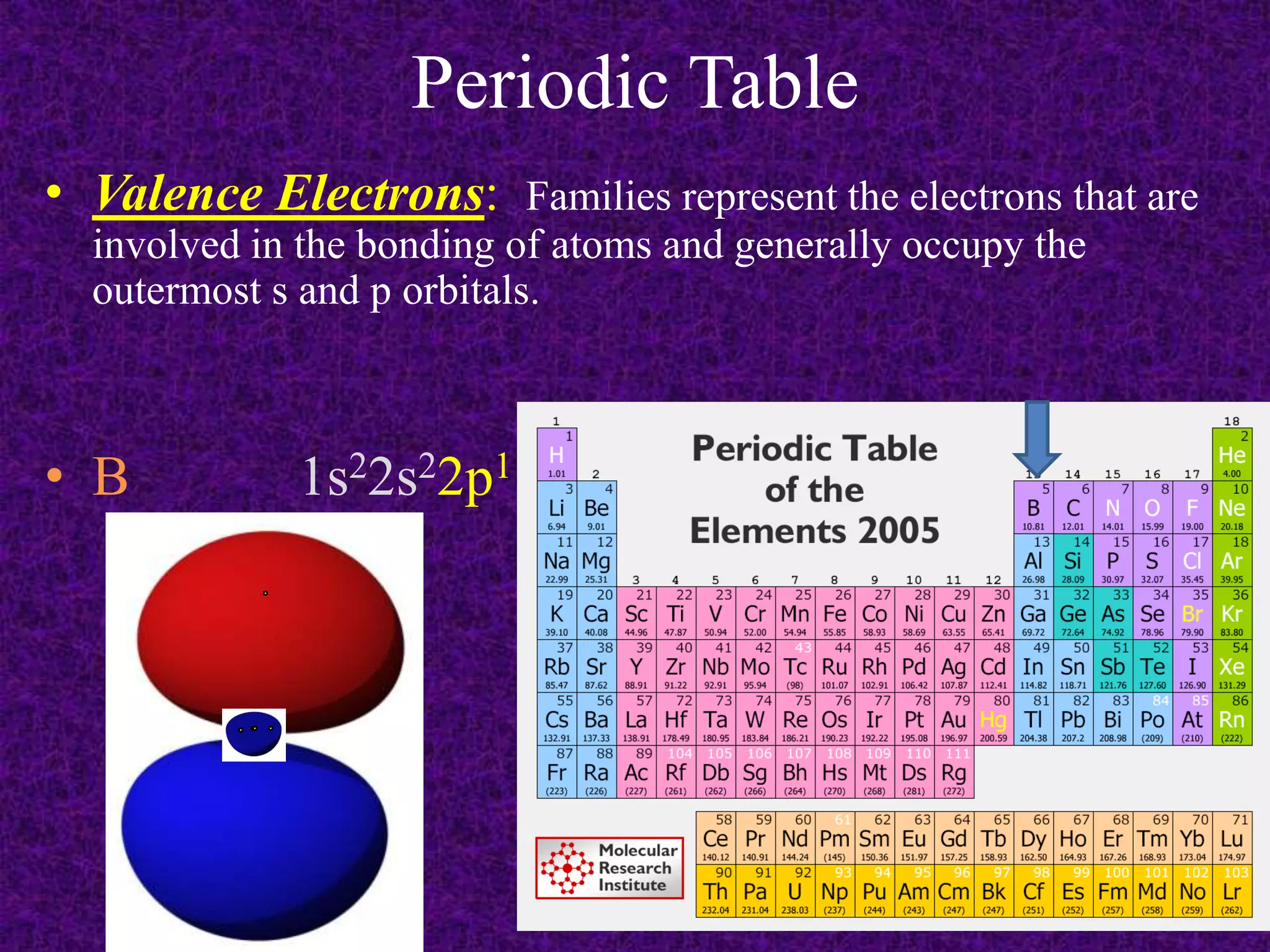
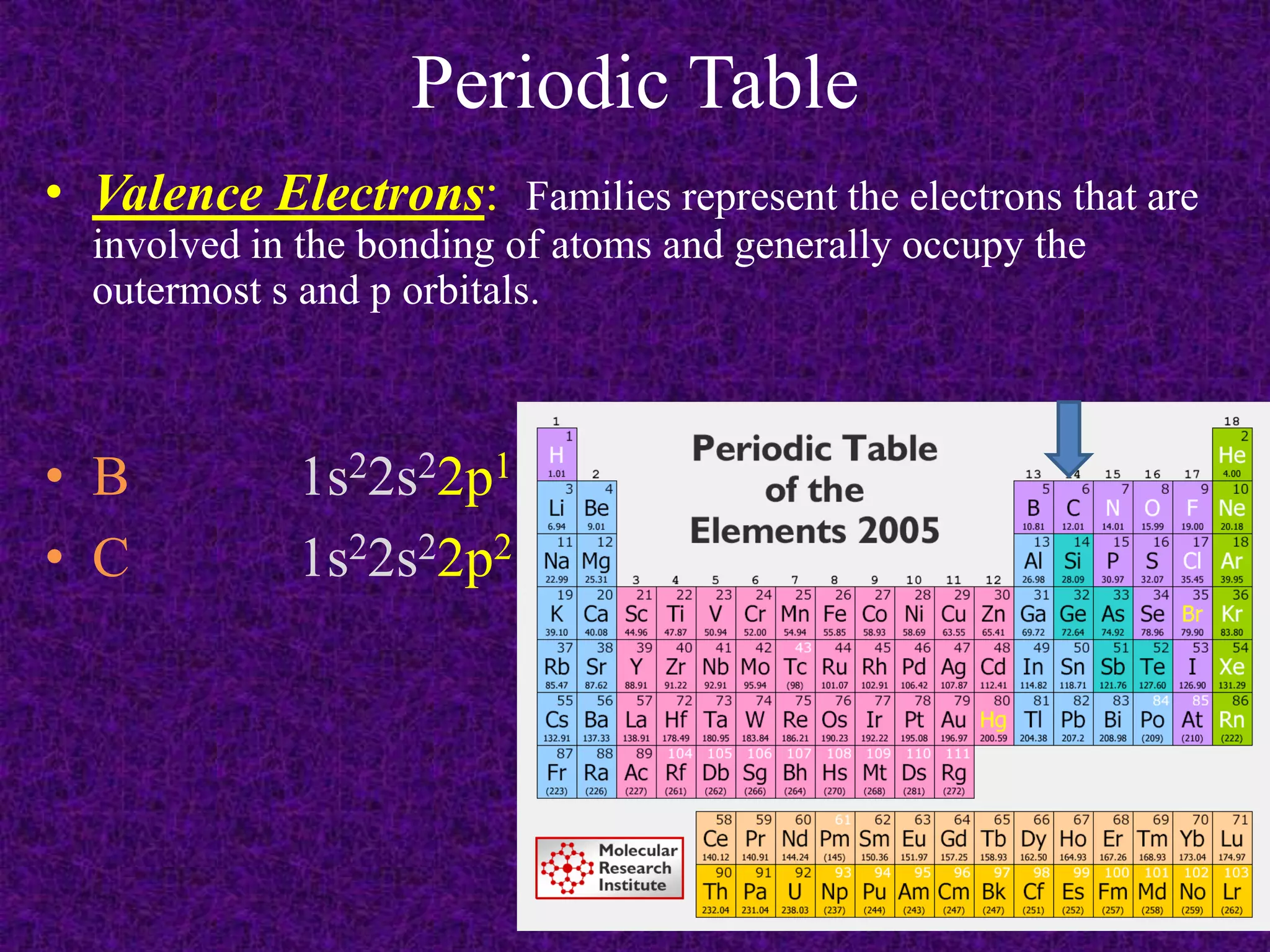
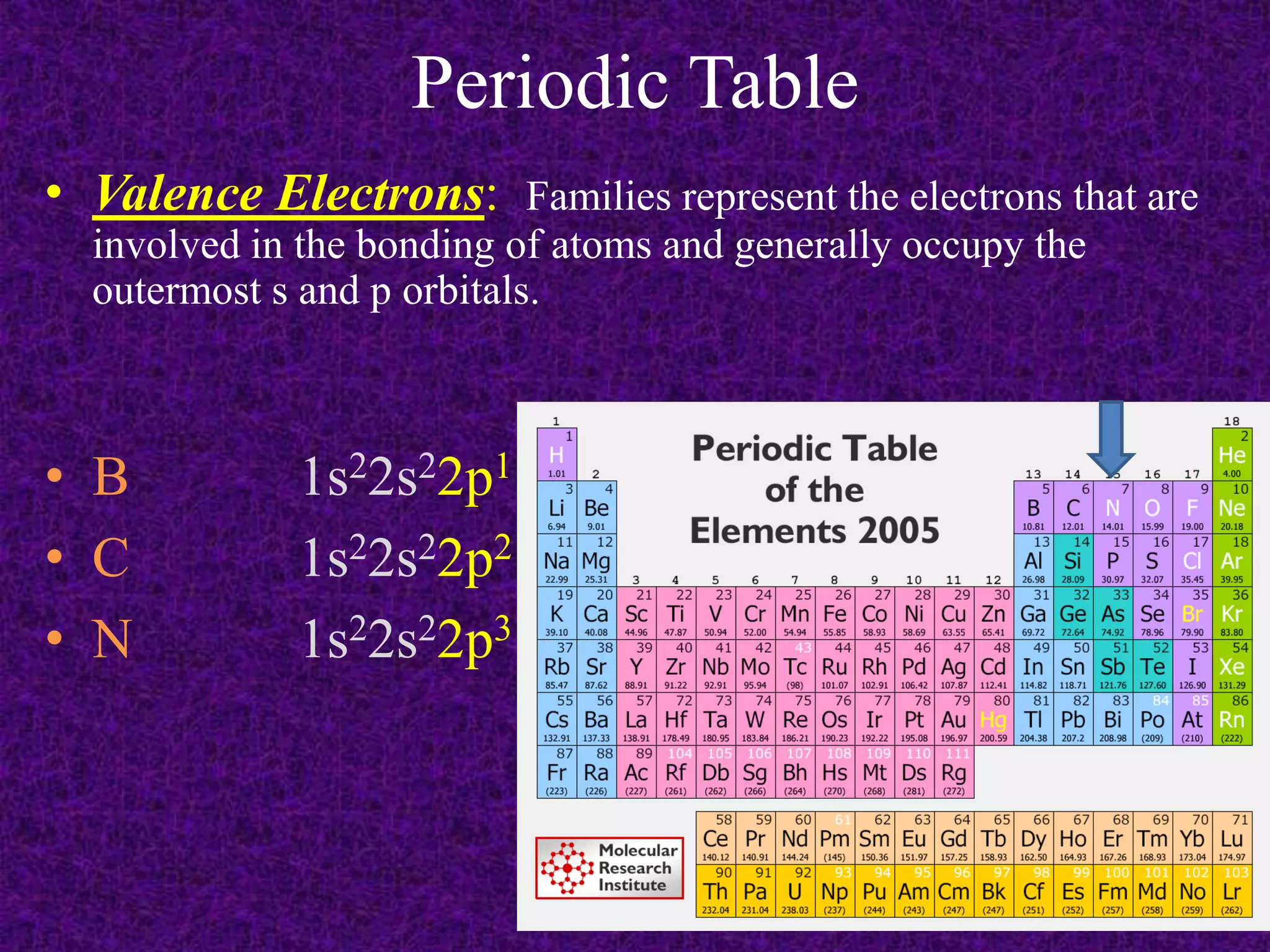
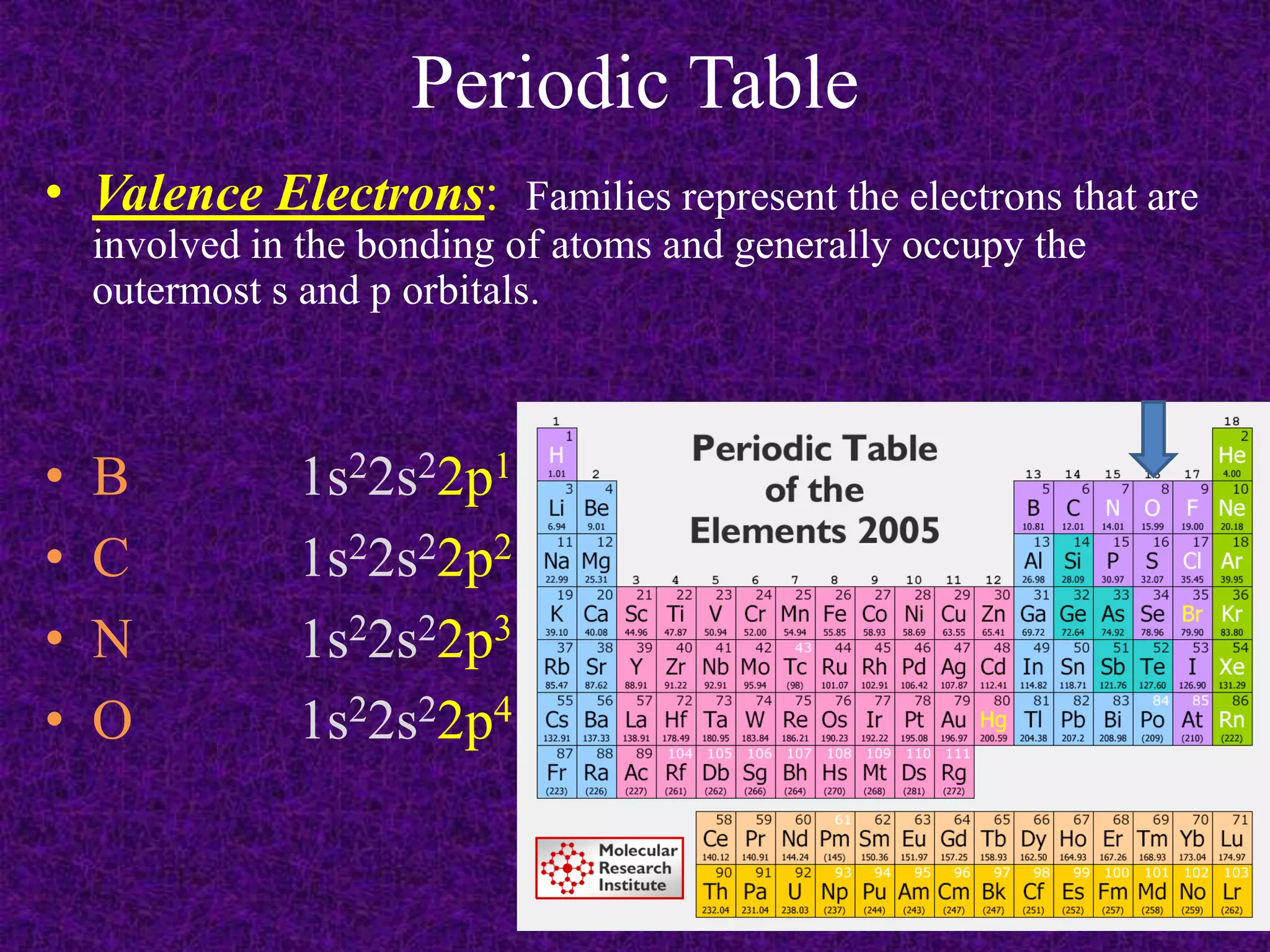
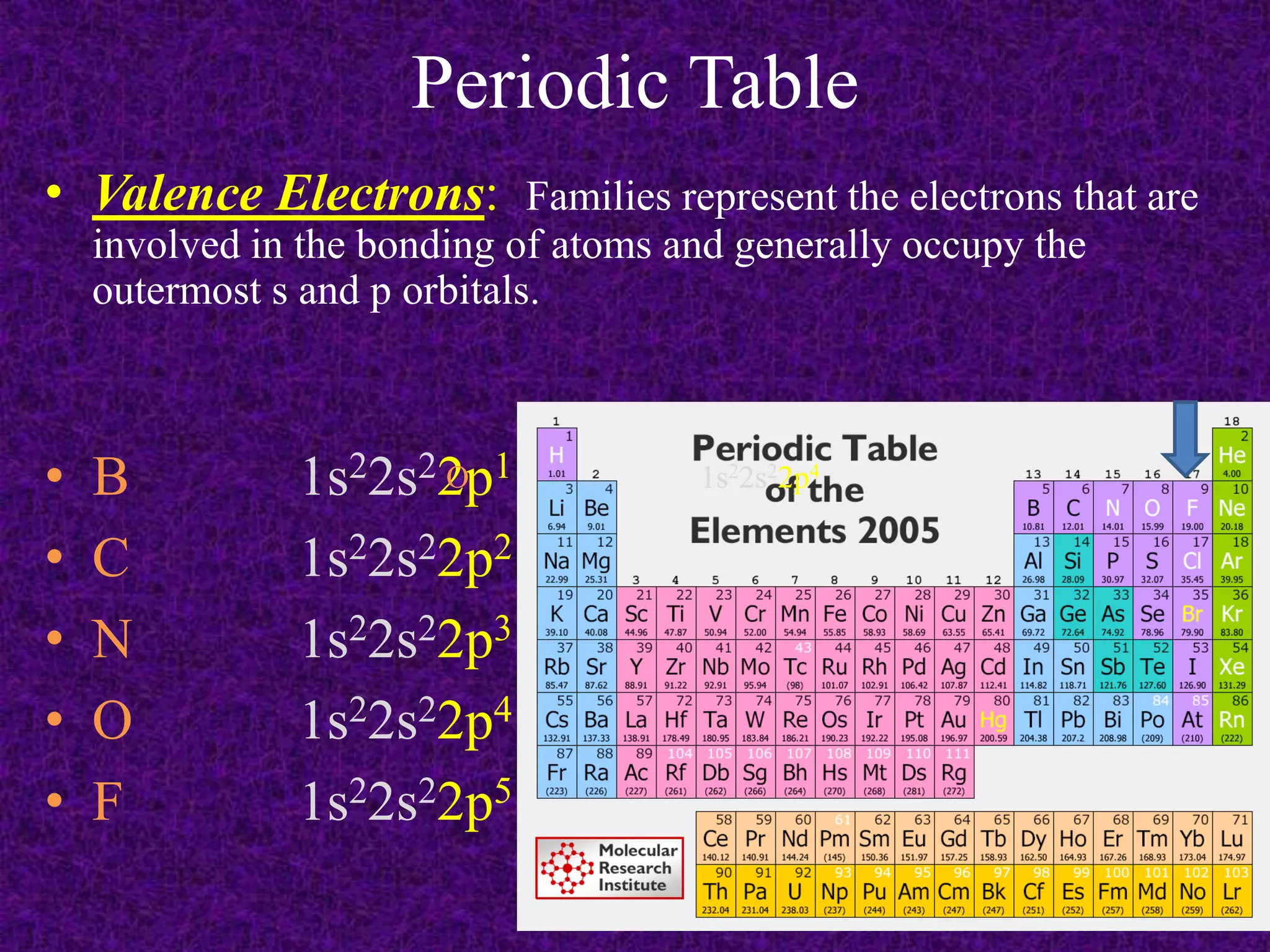
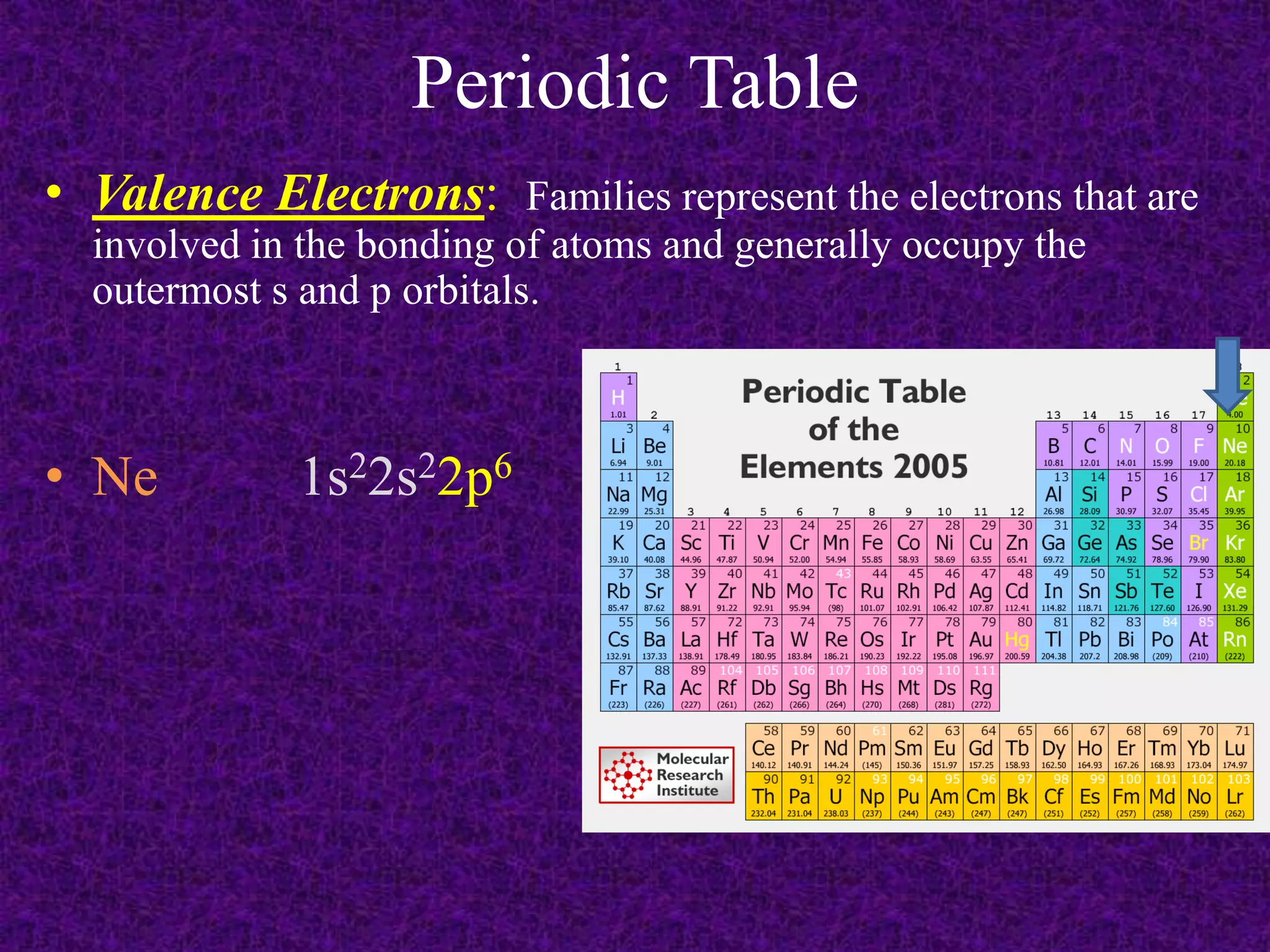
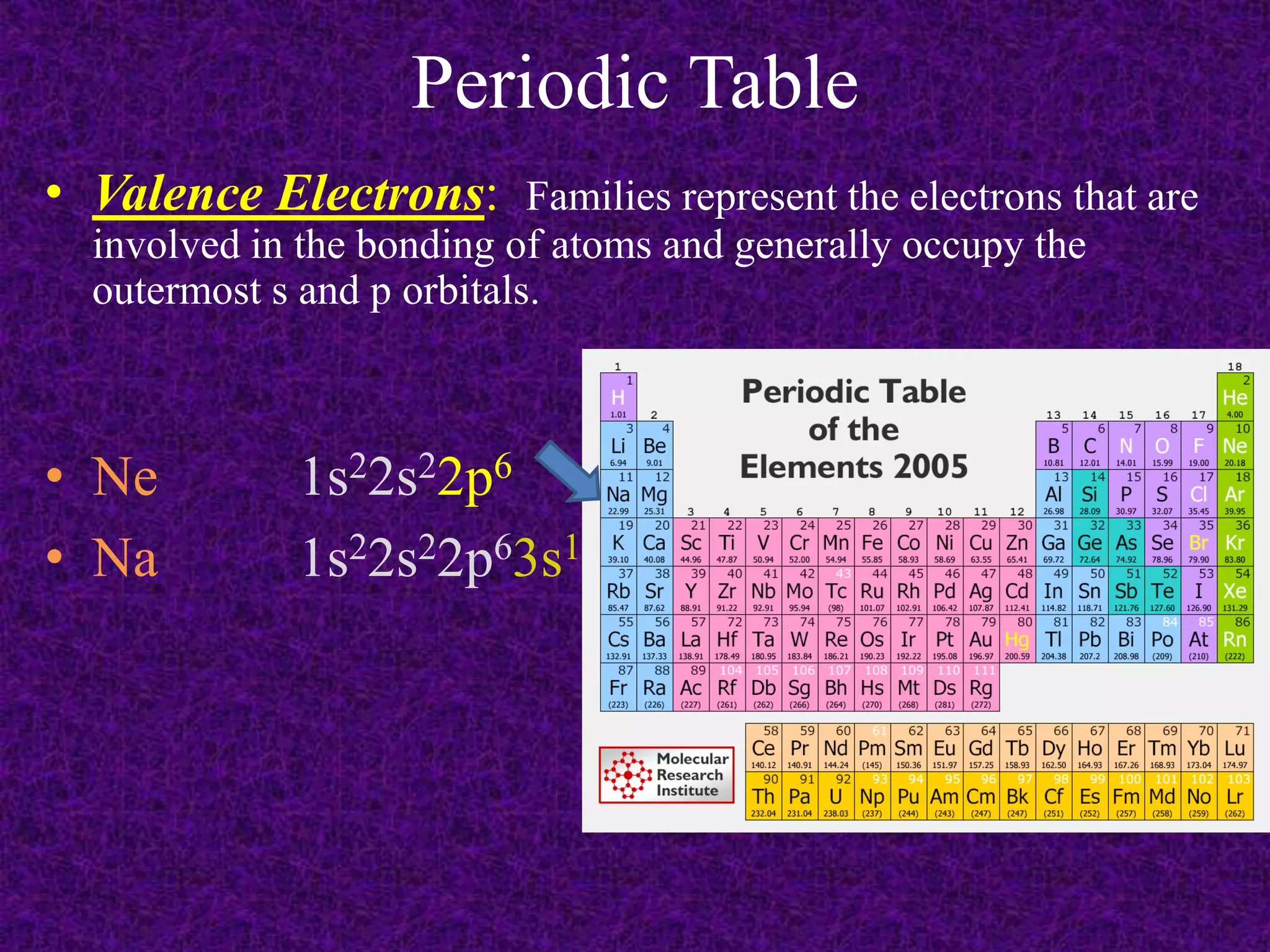
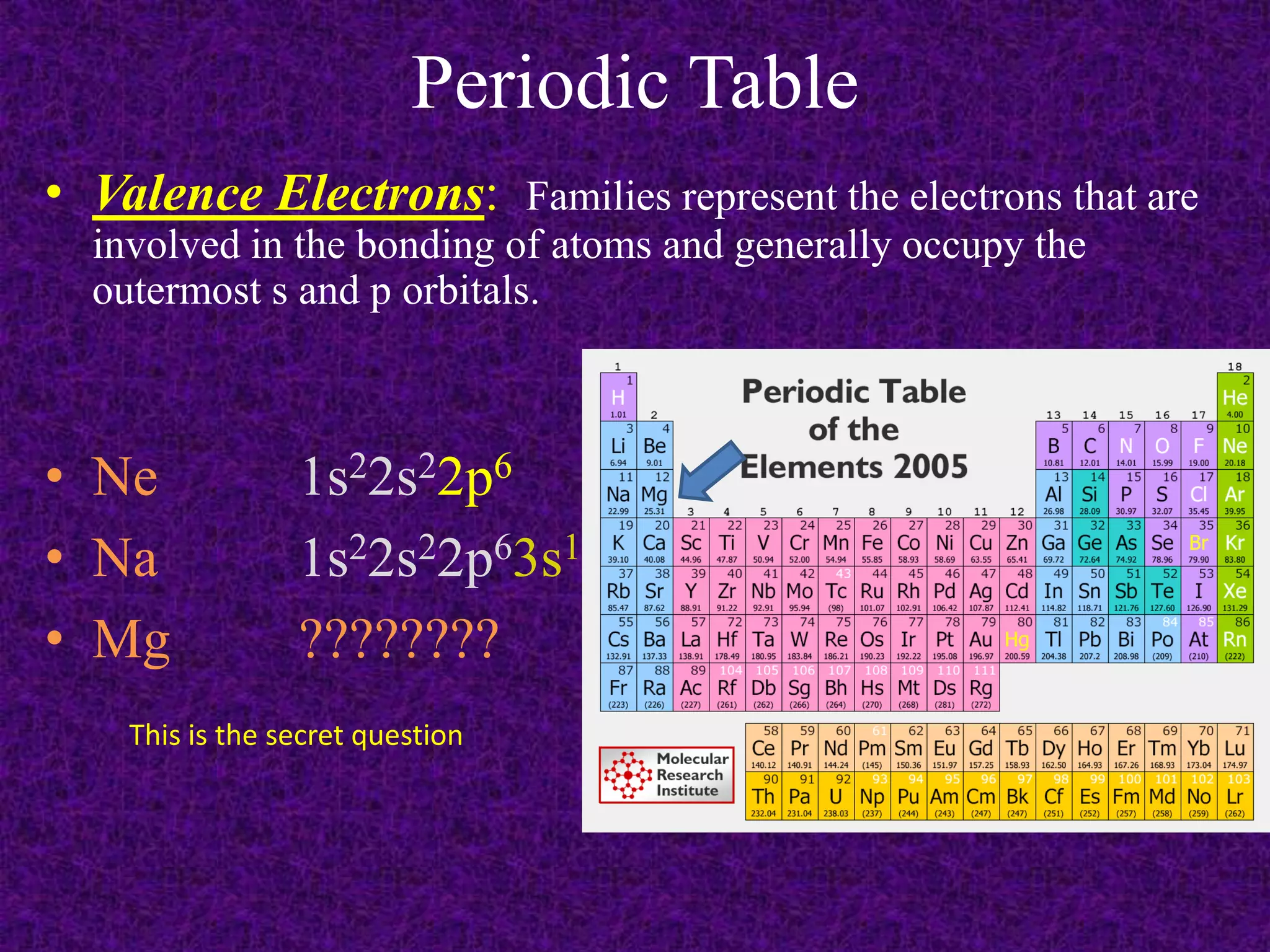
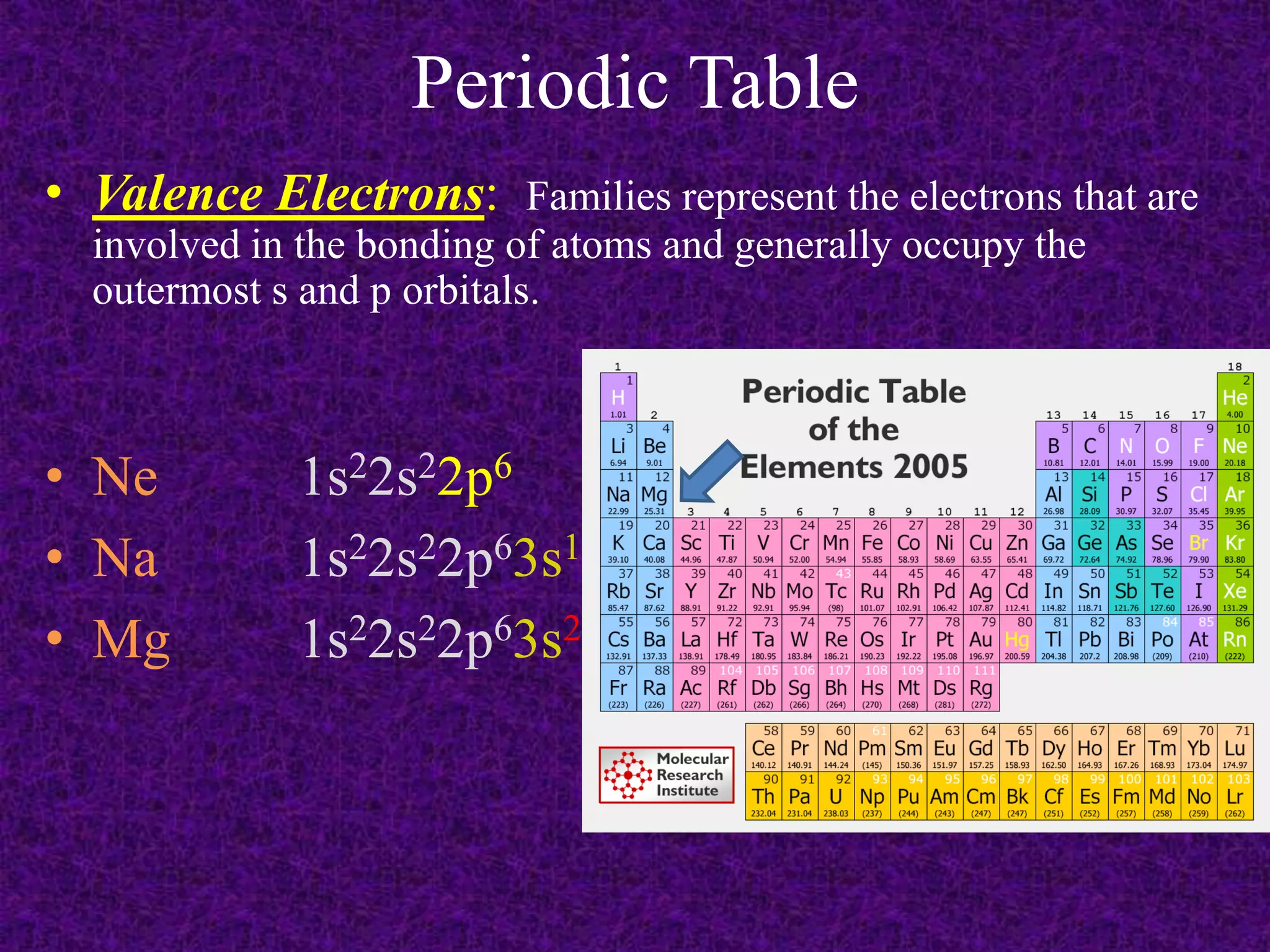
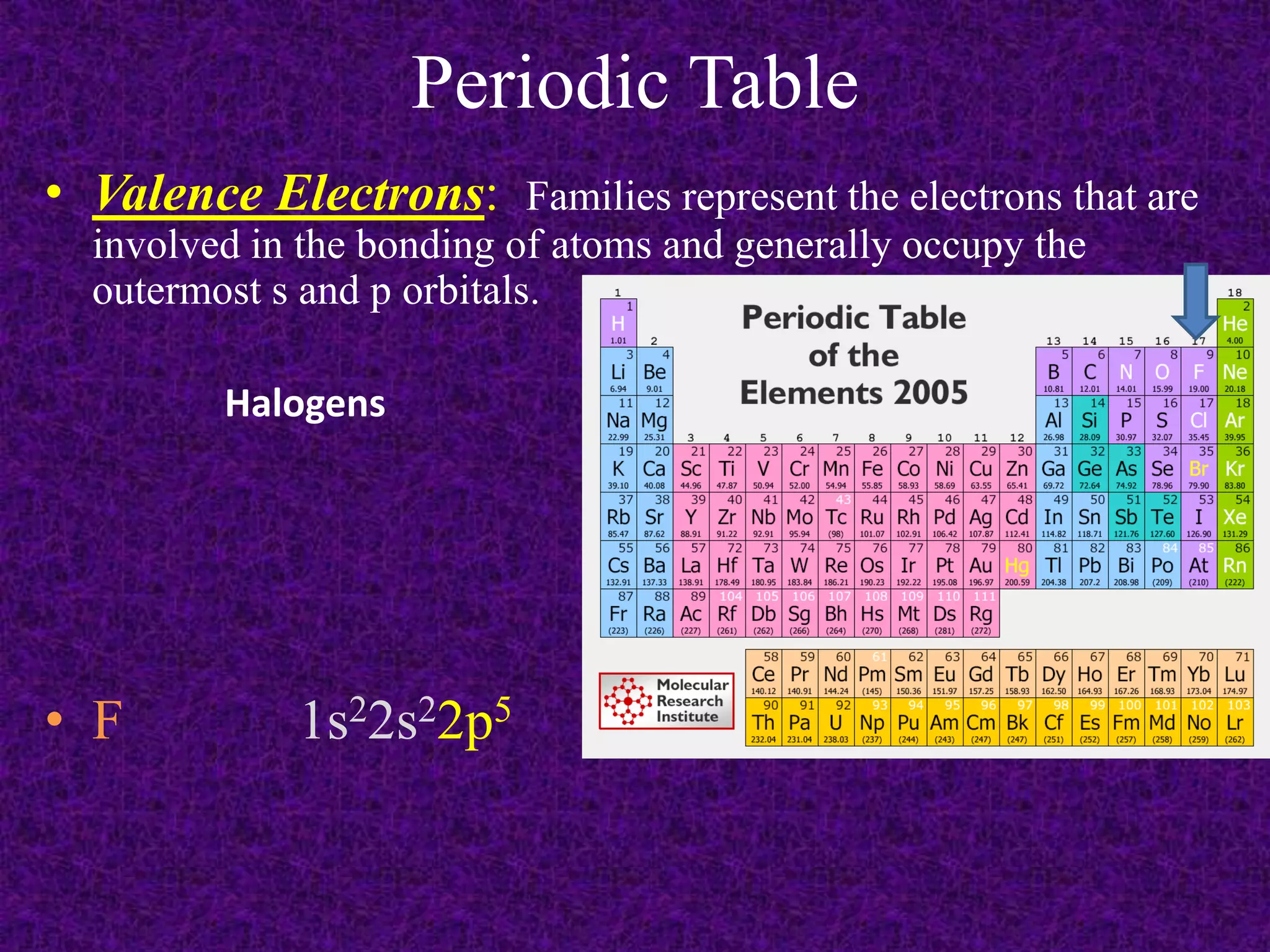
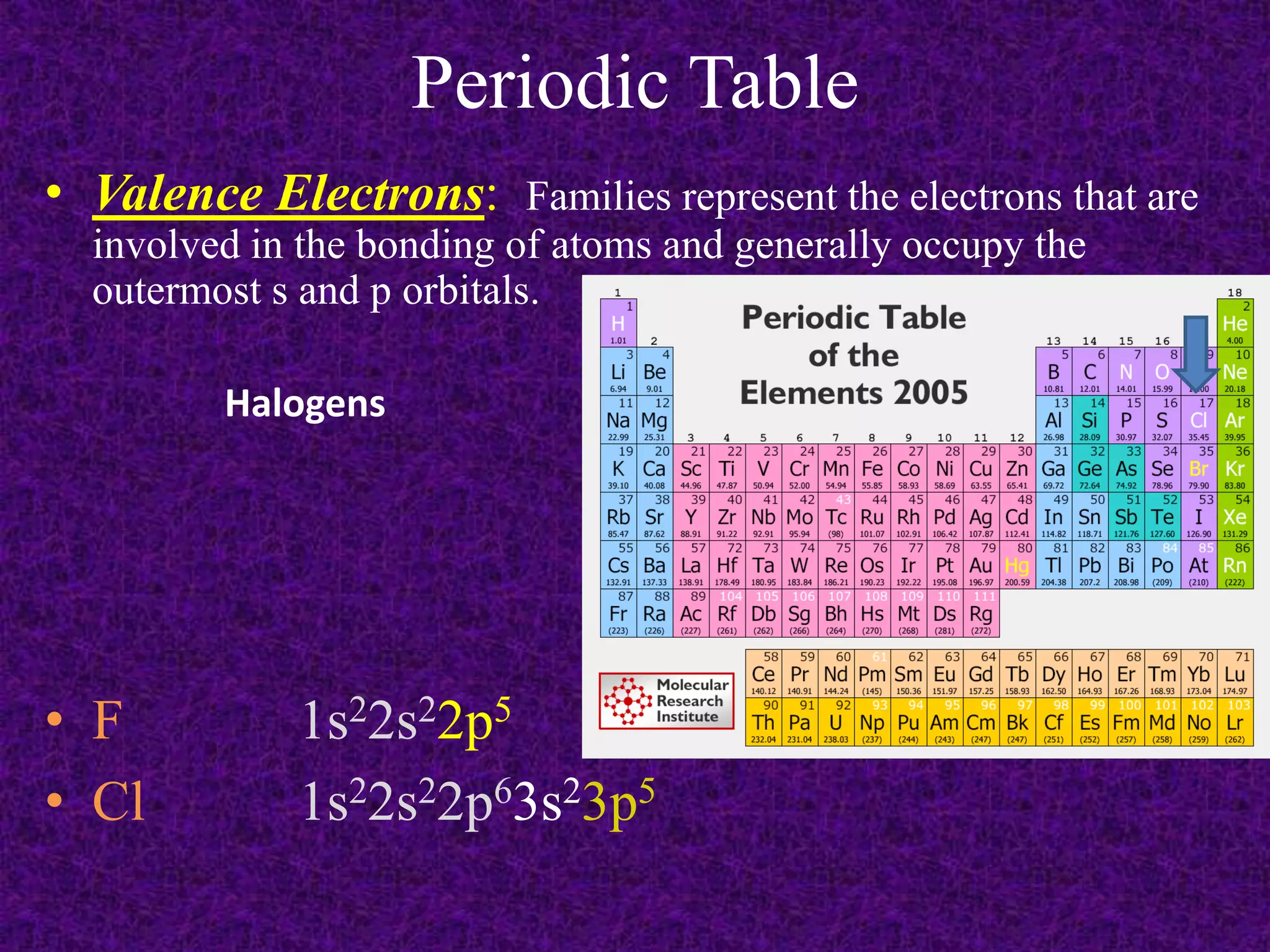
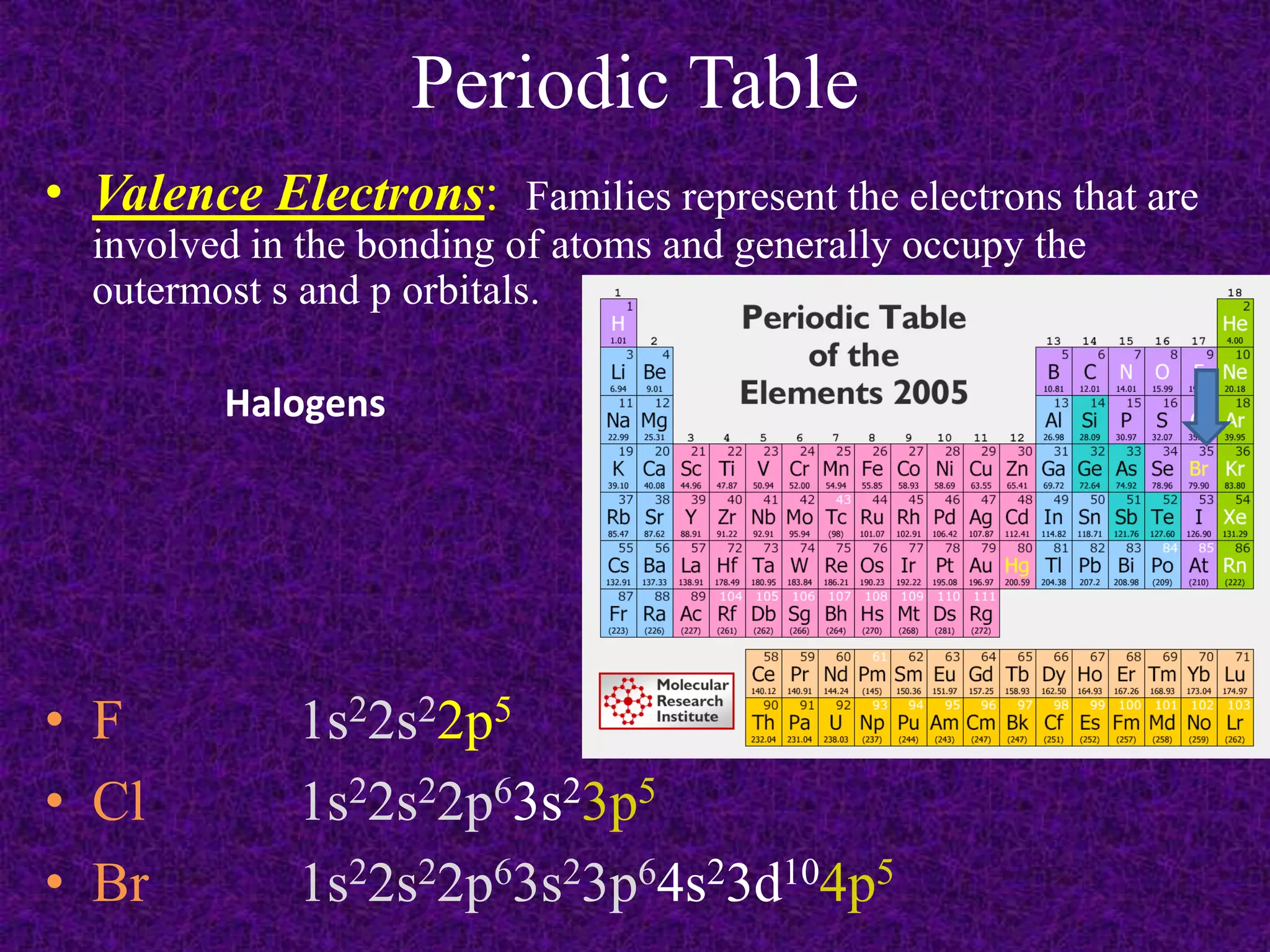
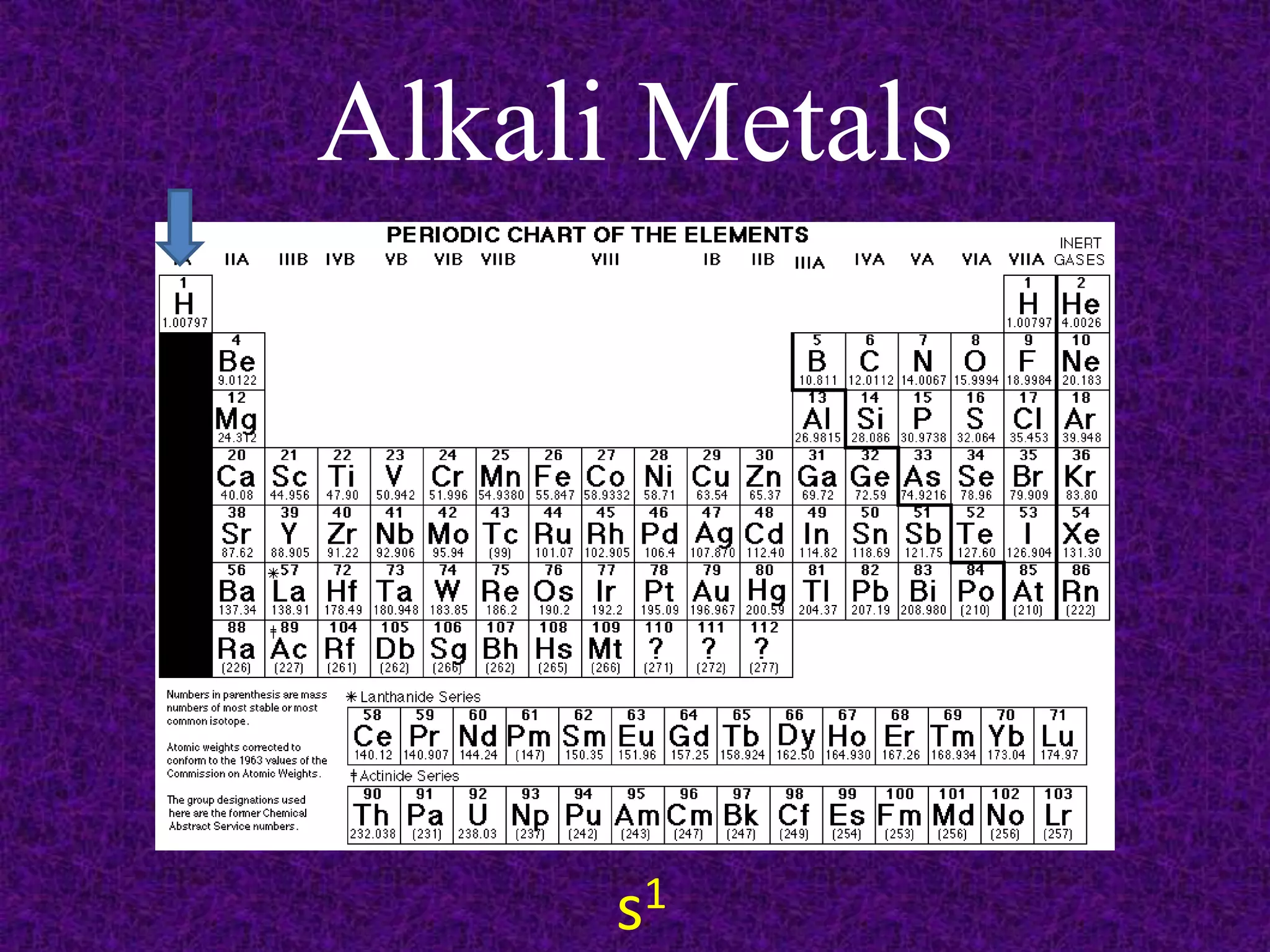
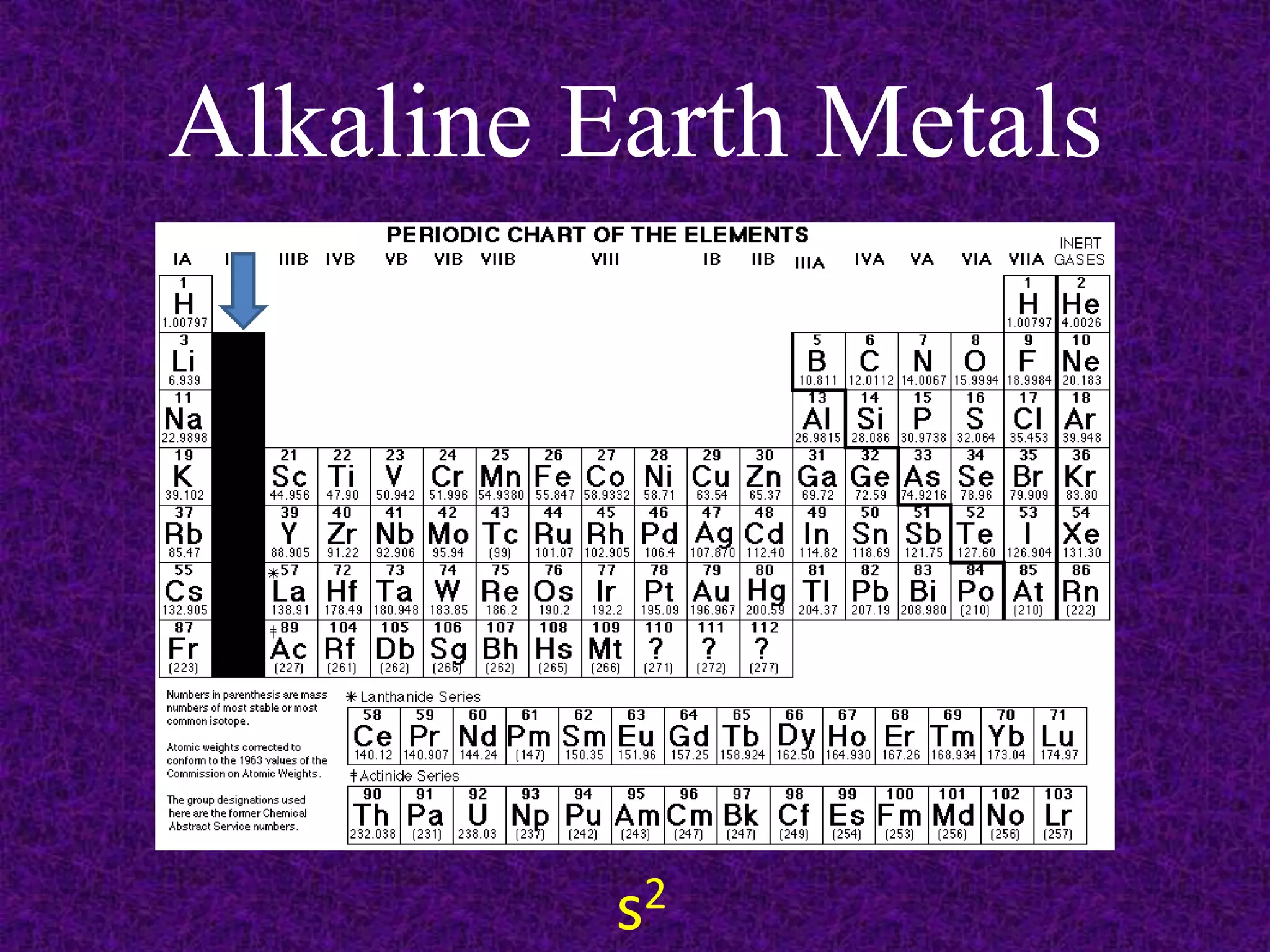
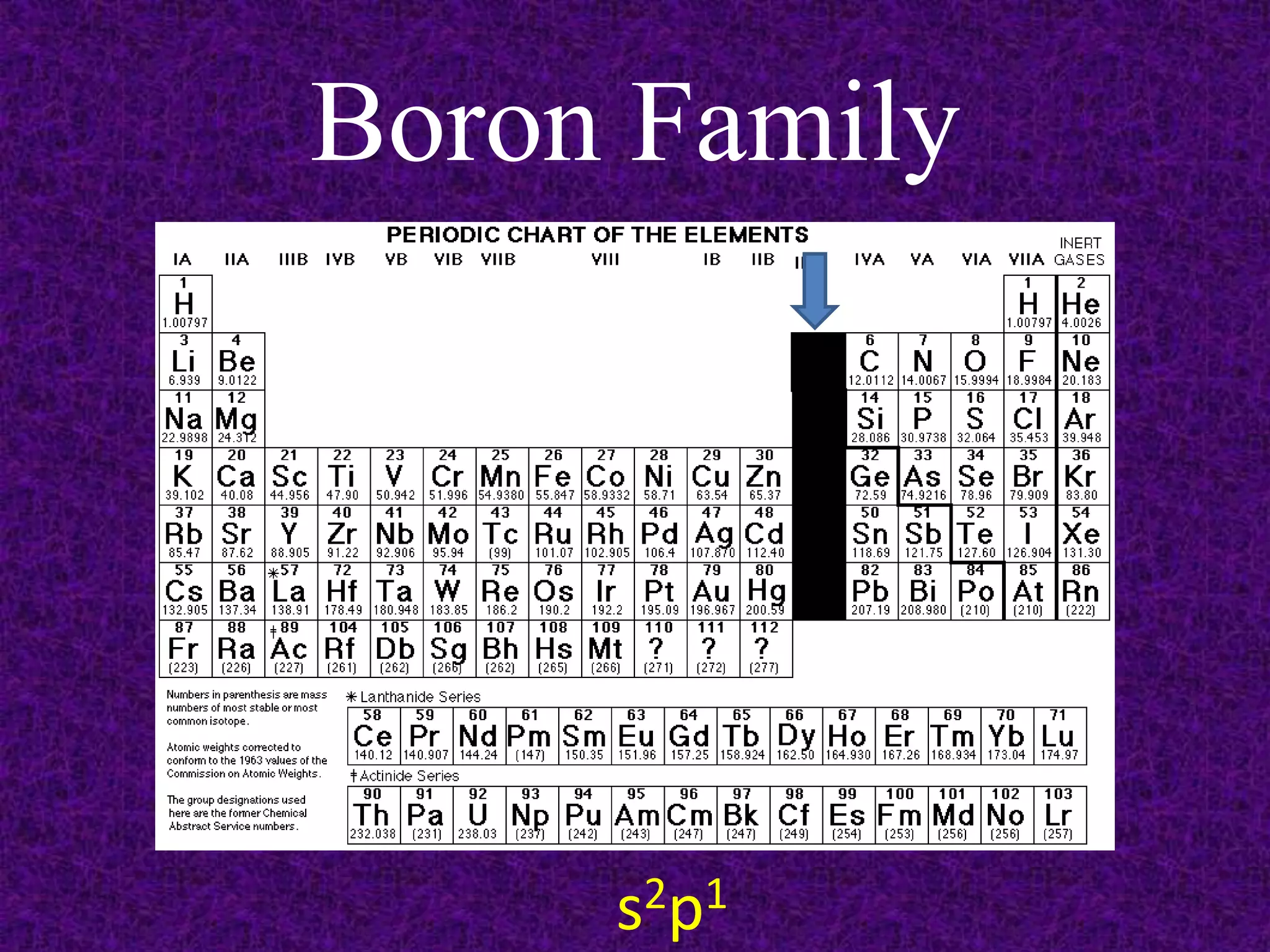
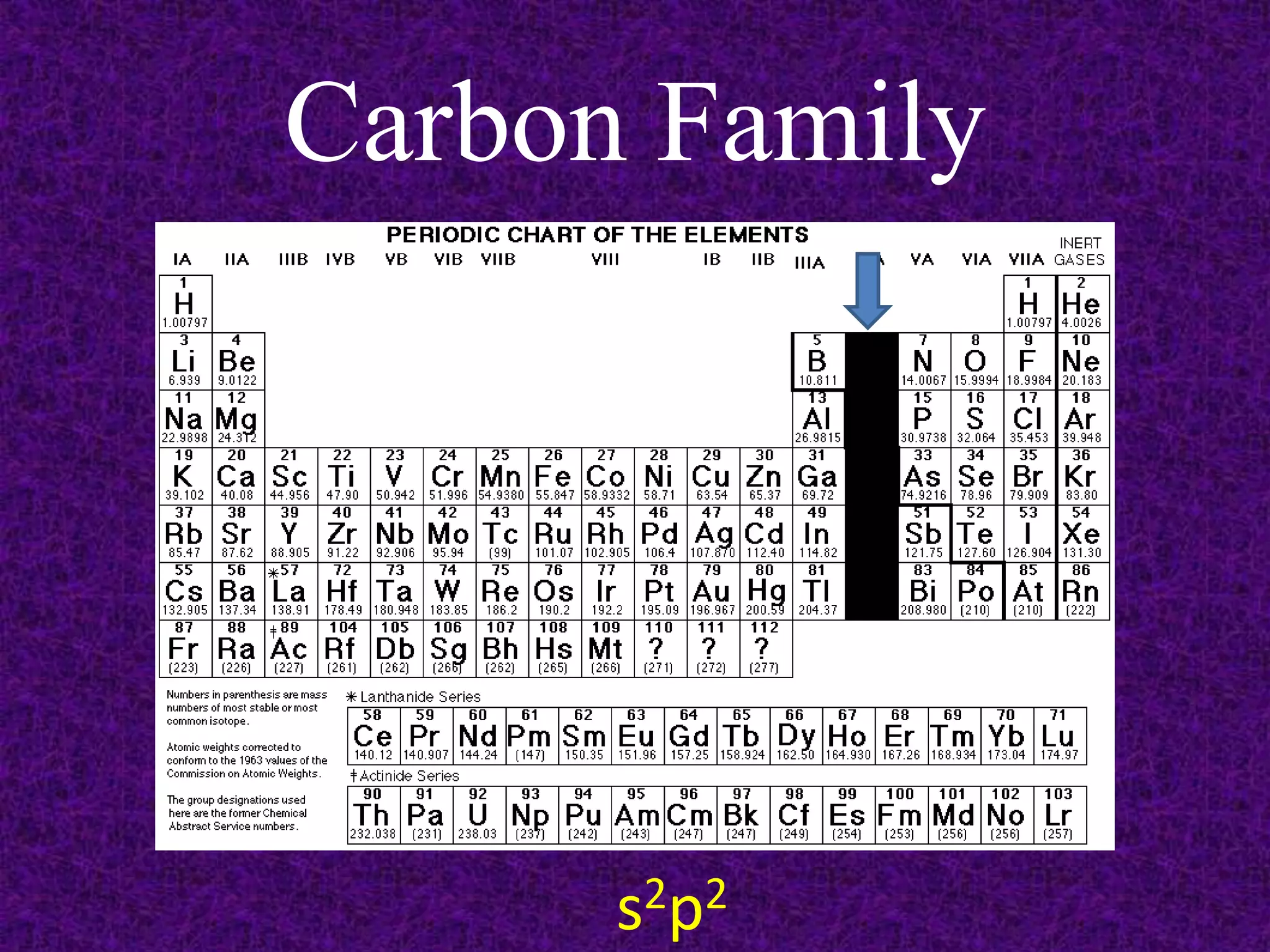
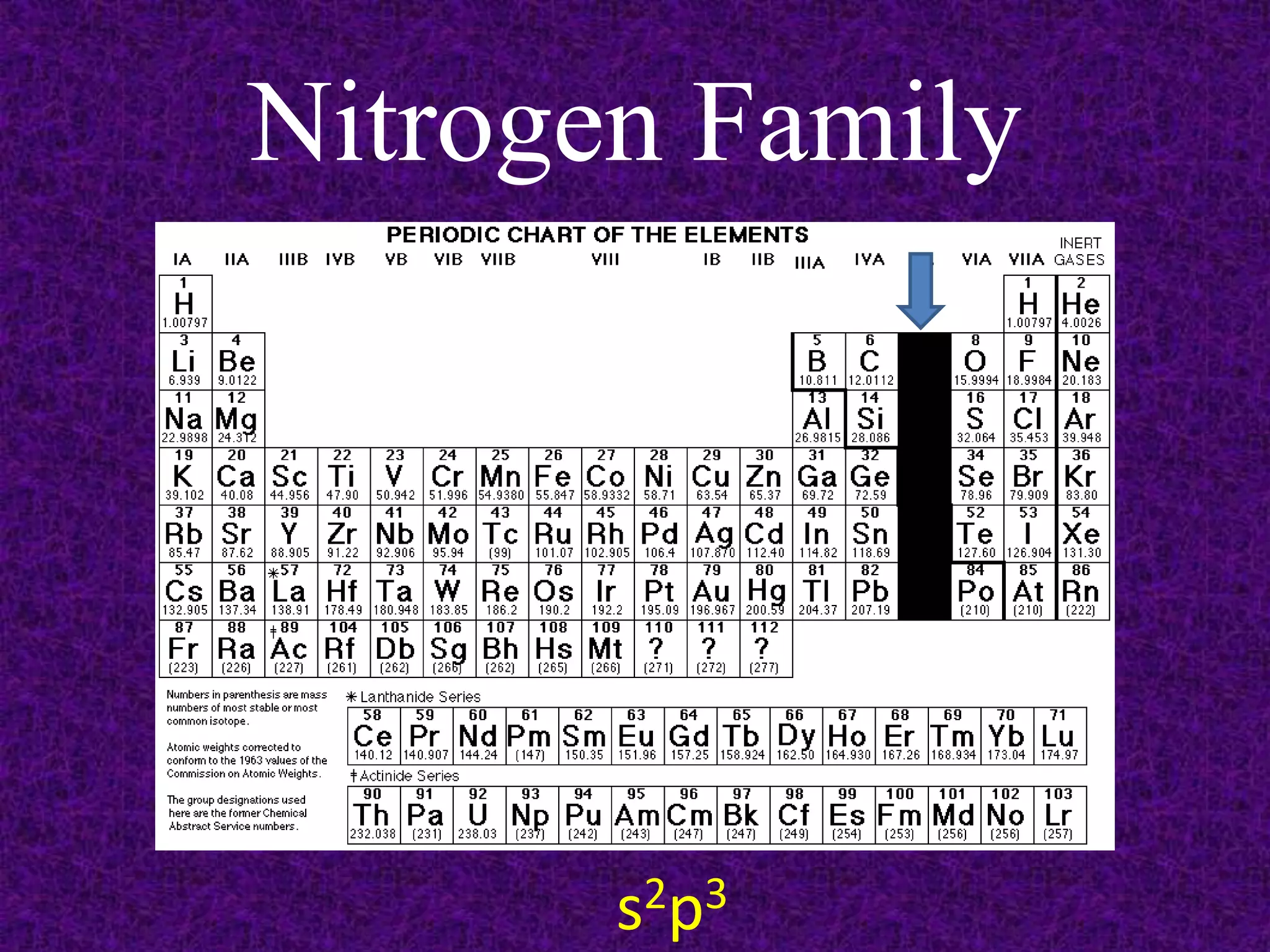
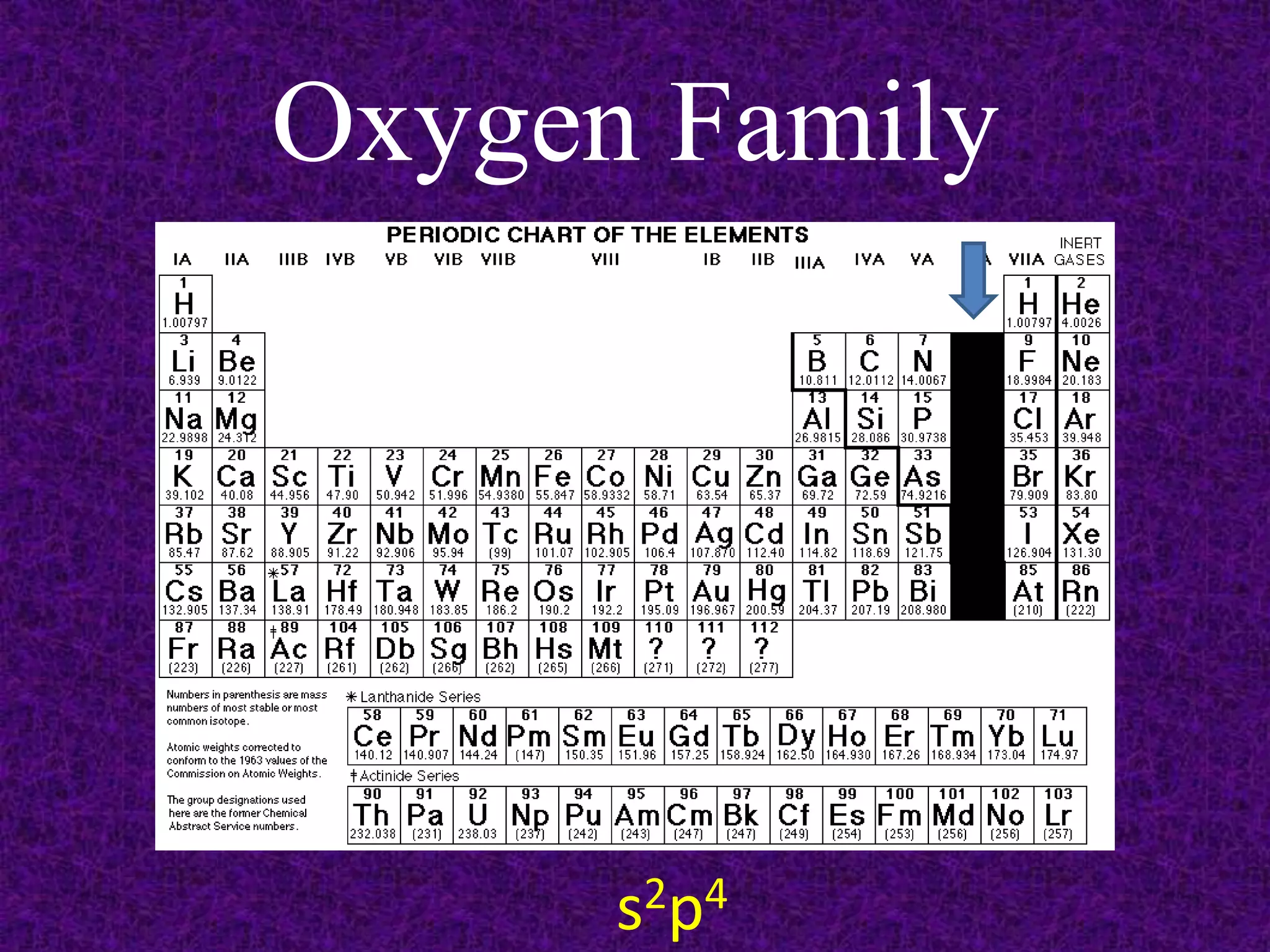
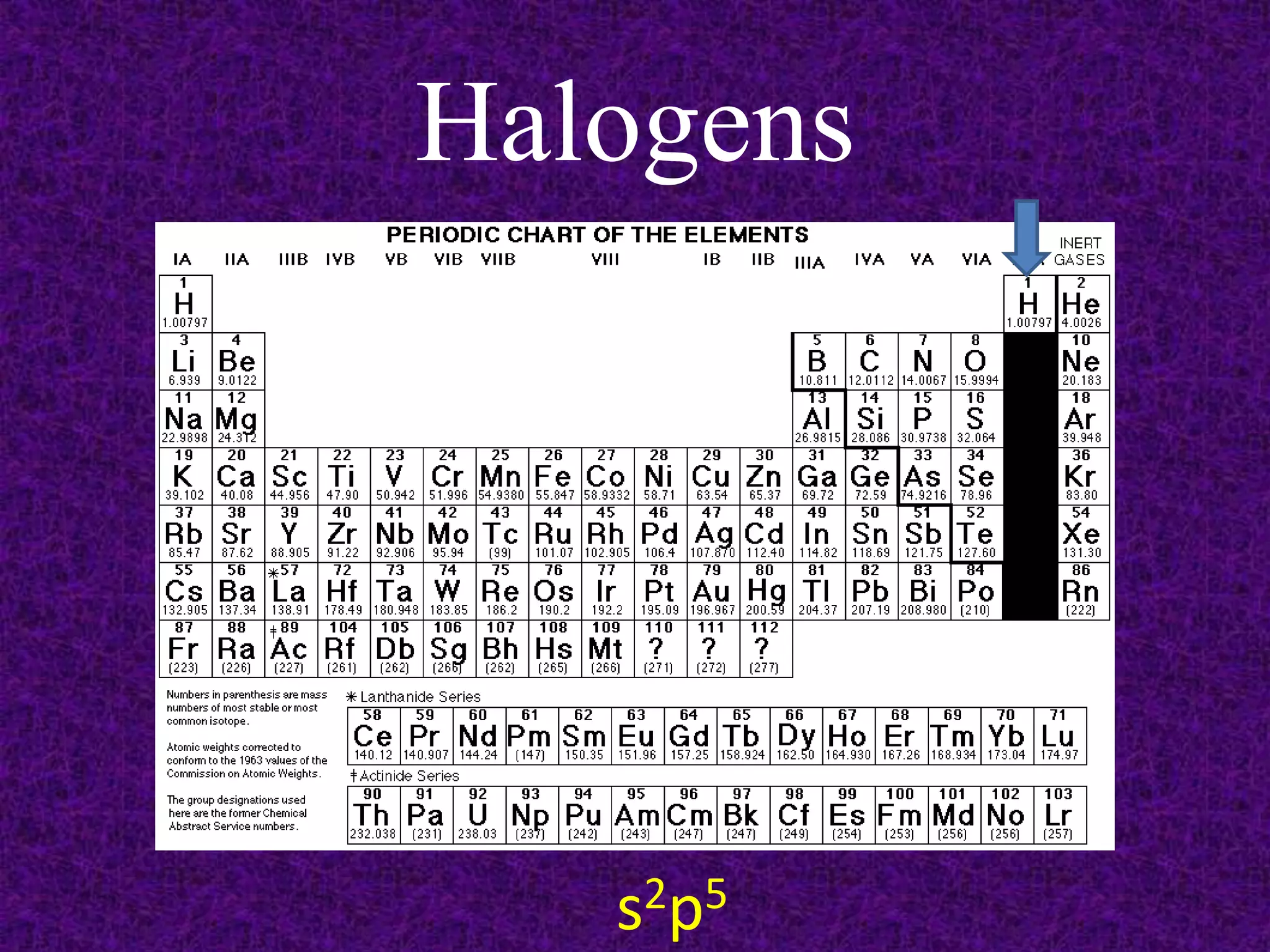
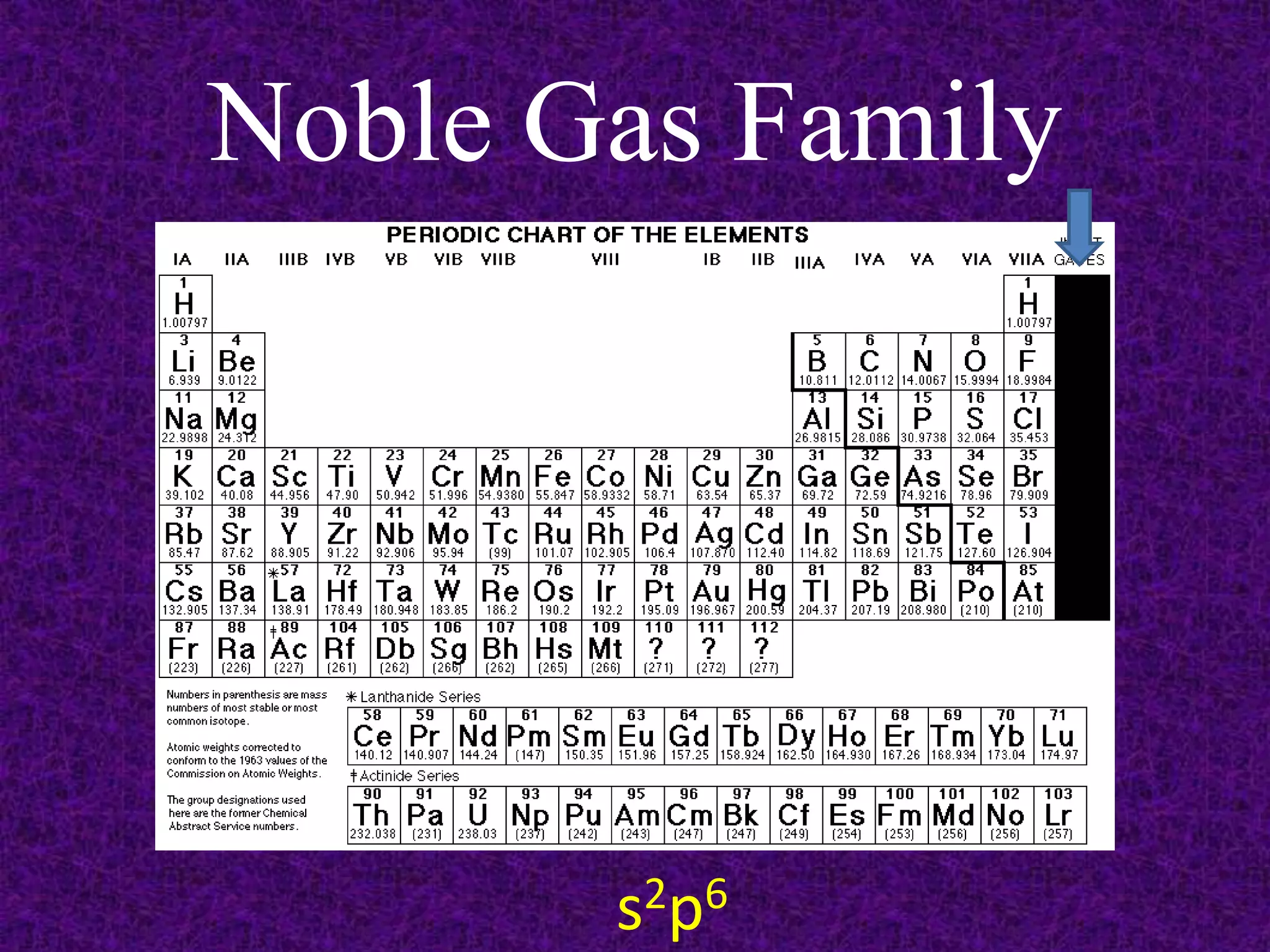
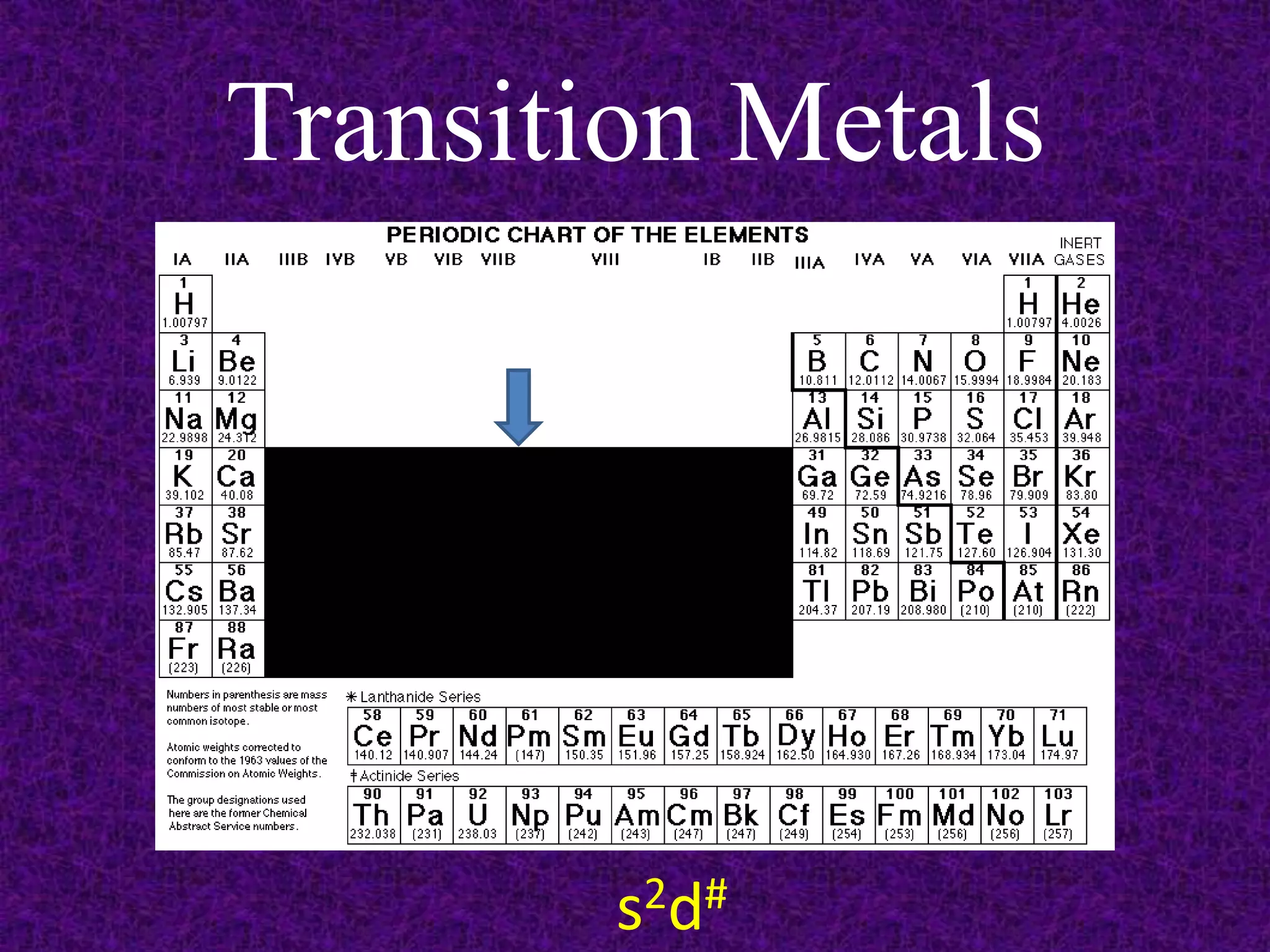
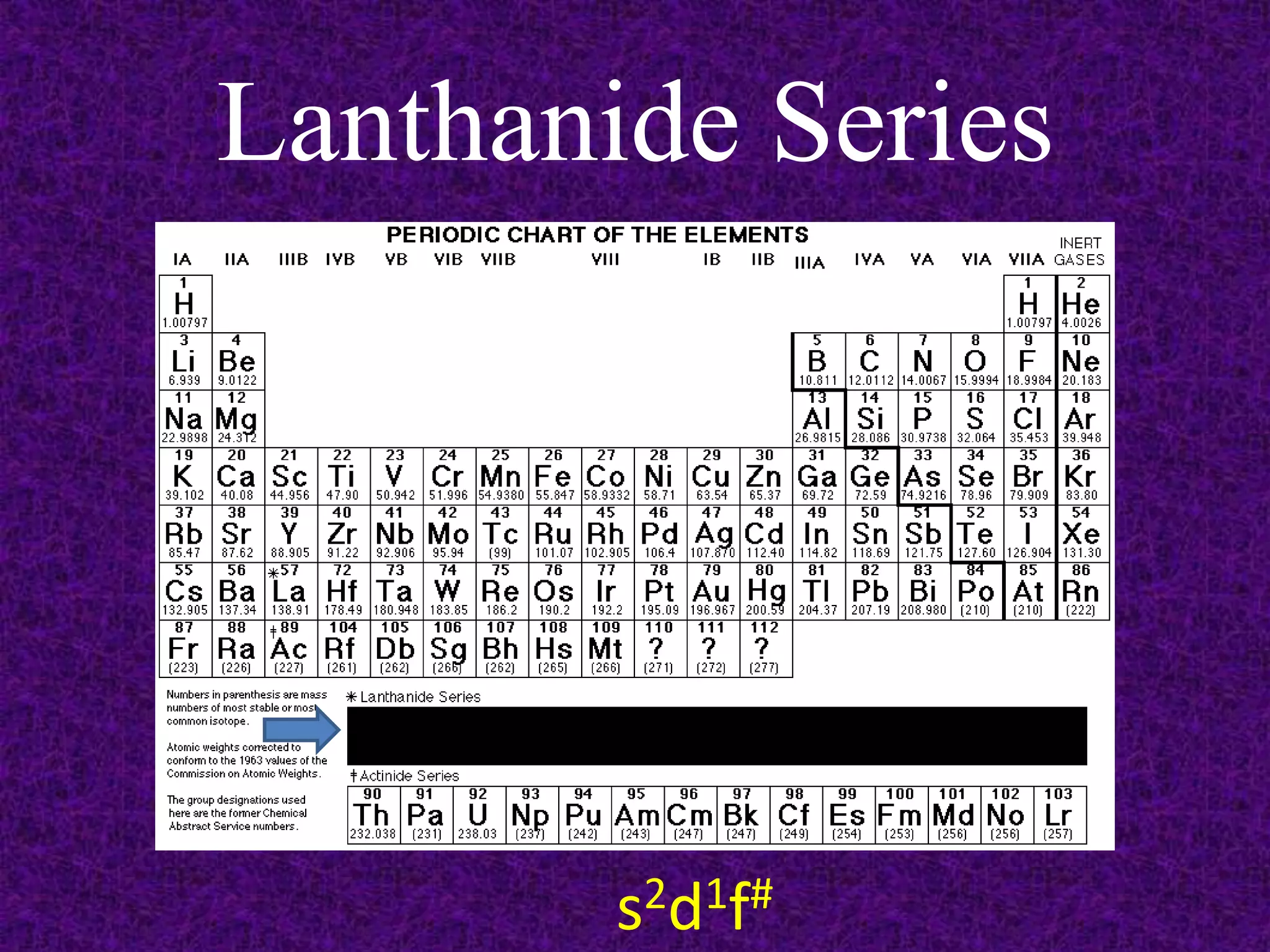
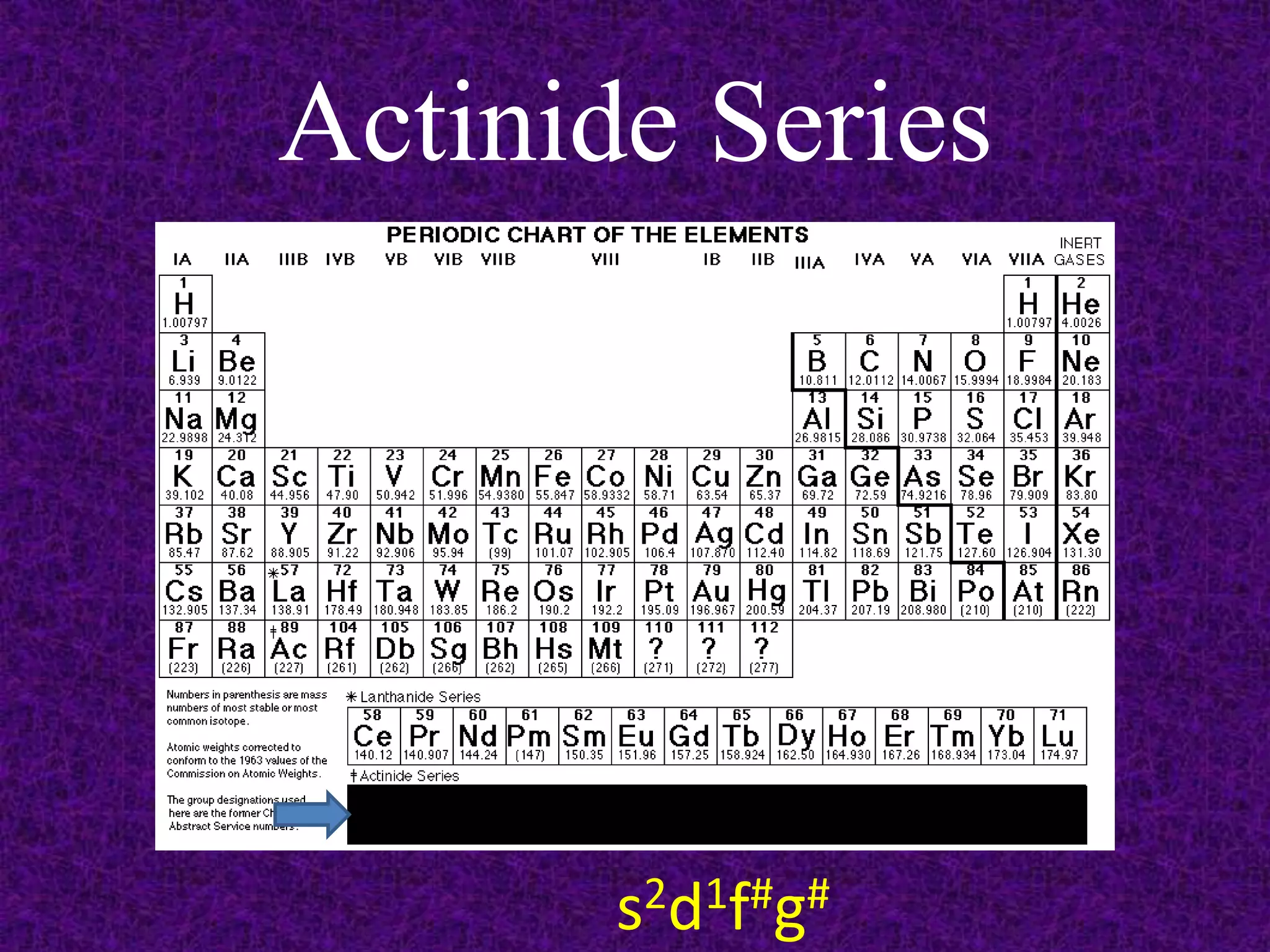
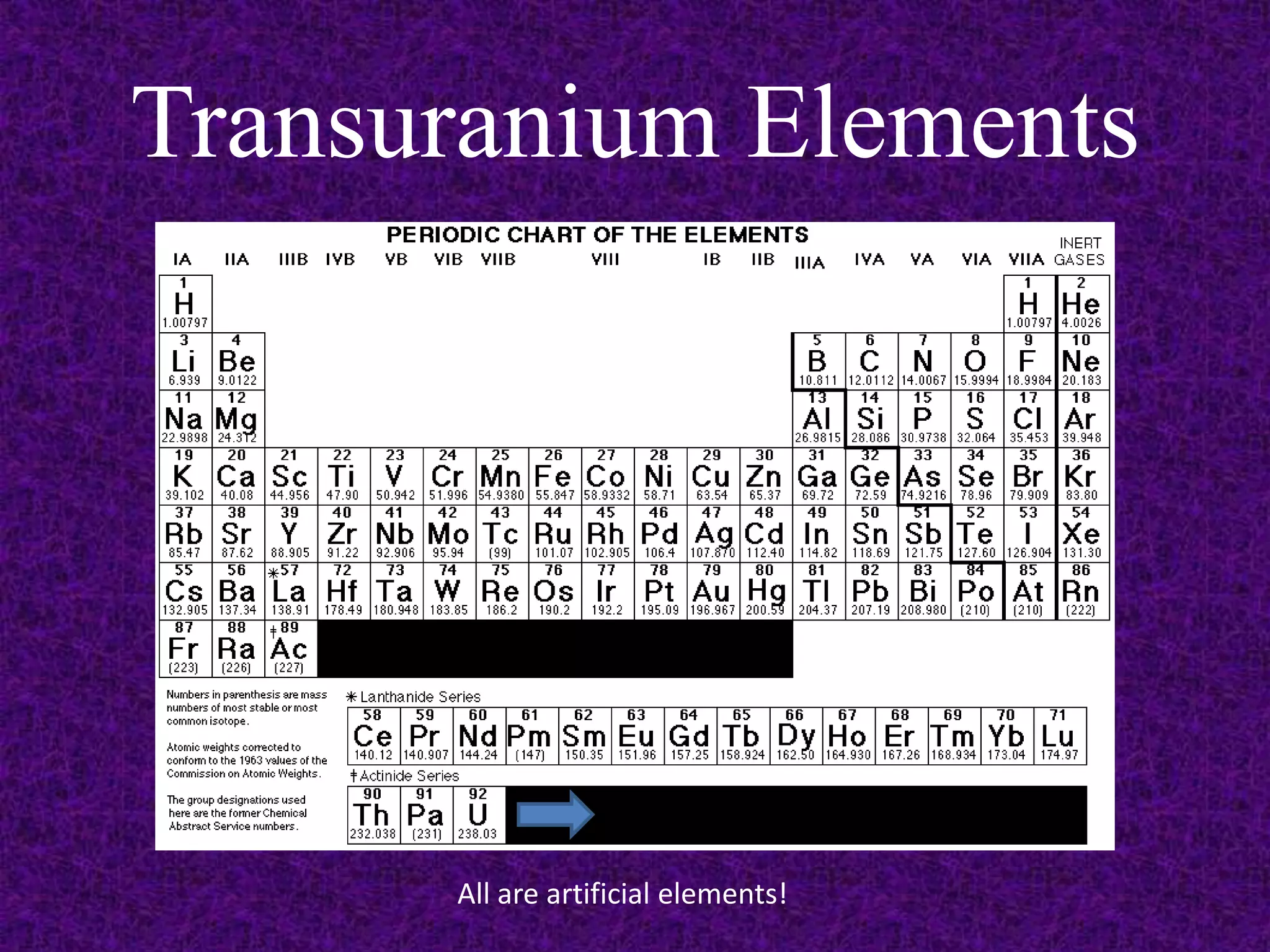
The document discusses the periodic table, including its organization into periods and families. It describes the electron configuration of elements in different families, showing how the outer electron shells determine an element's chemical properties and reactivity. Examples are given of elements from families such as alkali metals, halogens, and noble gases along with their electron configurations.