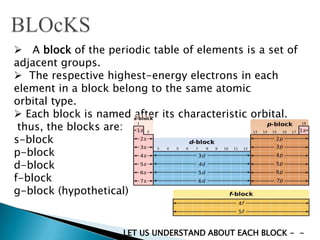
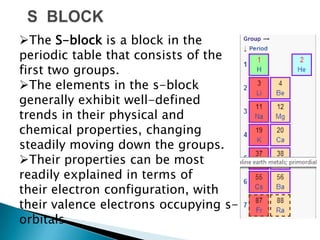
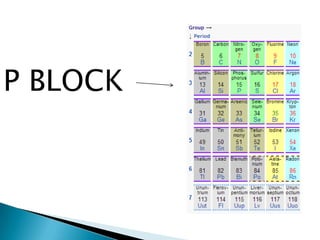
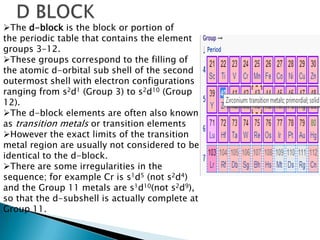
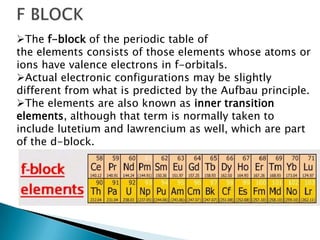
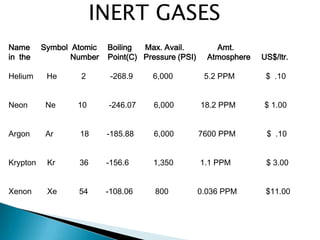
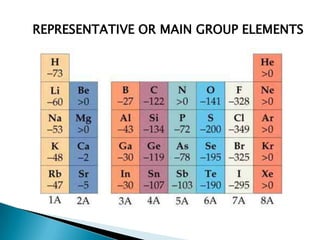
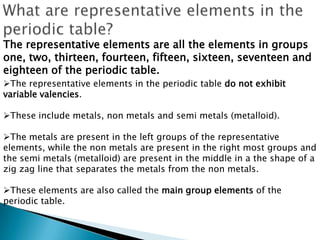
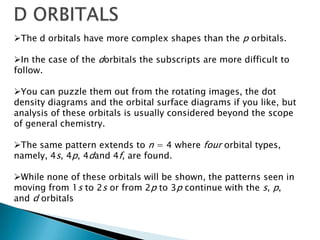
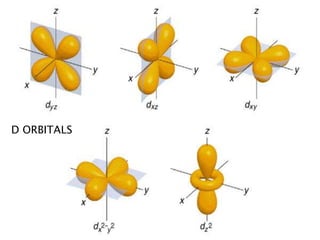
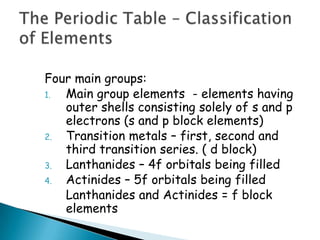
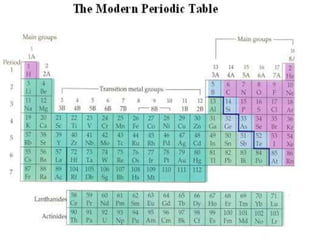
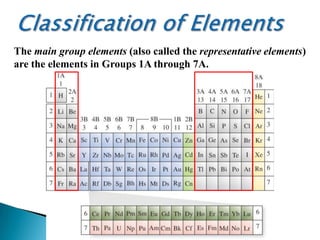
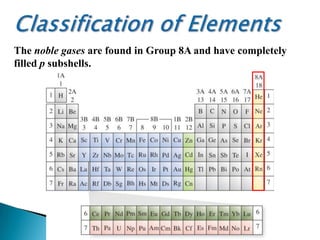
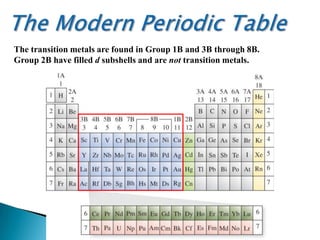
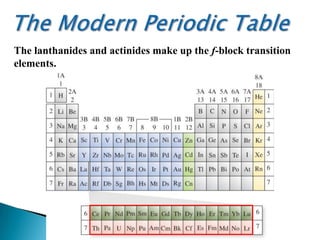
The document provides an overview of the periodic table, describing its organization by atomic number and the properties of elements grouped in various blocks (s, p, d, f). It outlines the characteristics of each block and the classification of elements, including main group elements and transition metals. Additionally, it discusses inert gases and atomic orbitals, explaining their significance in the periodic structure and behavior of elements.

![There is a distinct pattern to the electron
configurations of the elements in a particular group.
For Group 1A: [noble gas]ns1 For Group 2A: [noble gas]ns2](https://image.slidesharecdn.com/classificationofelements-140315060054-phpapp02/85/Classification-of-Elements-Powerpoint-Presentation-by-Computer-Careers-10-320.jpg)