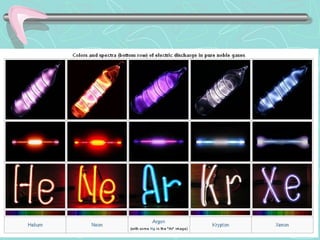
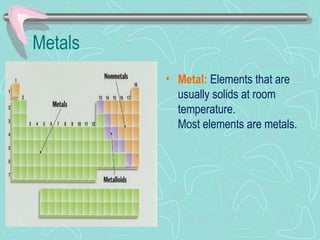
The document provides information about the periodic table. It discusses how Dmitri Mendeleev created one of the first periodic tables by organizing elements by atomic mass. It also explains how the modern periodic table arranges elements by atomic number and divides them into periods and groups. Key aspects of the periodic table like metals, nonmetals, periods, groups, and families of elements are defined and examples are given.