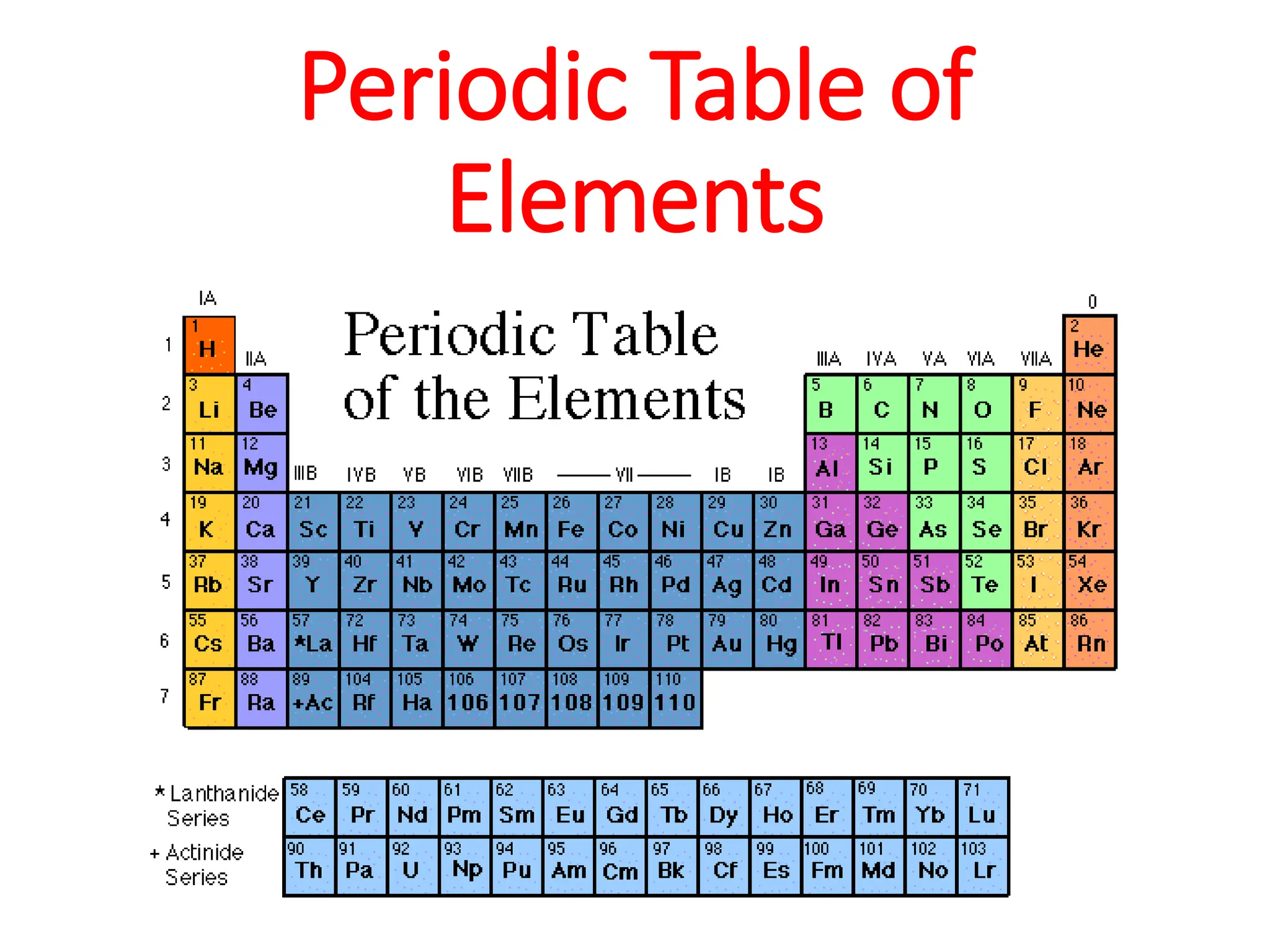
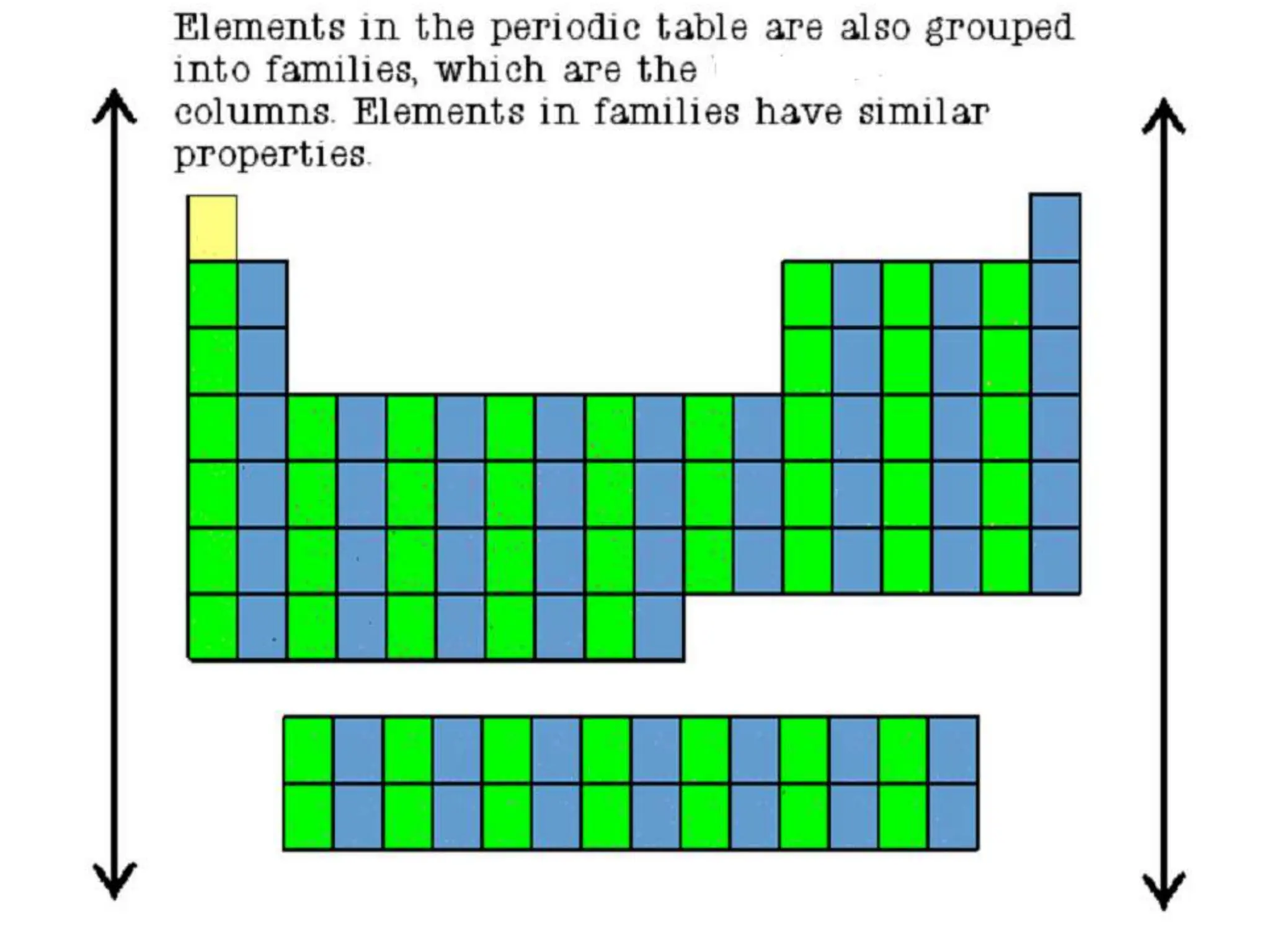
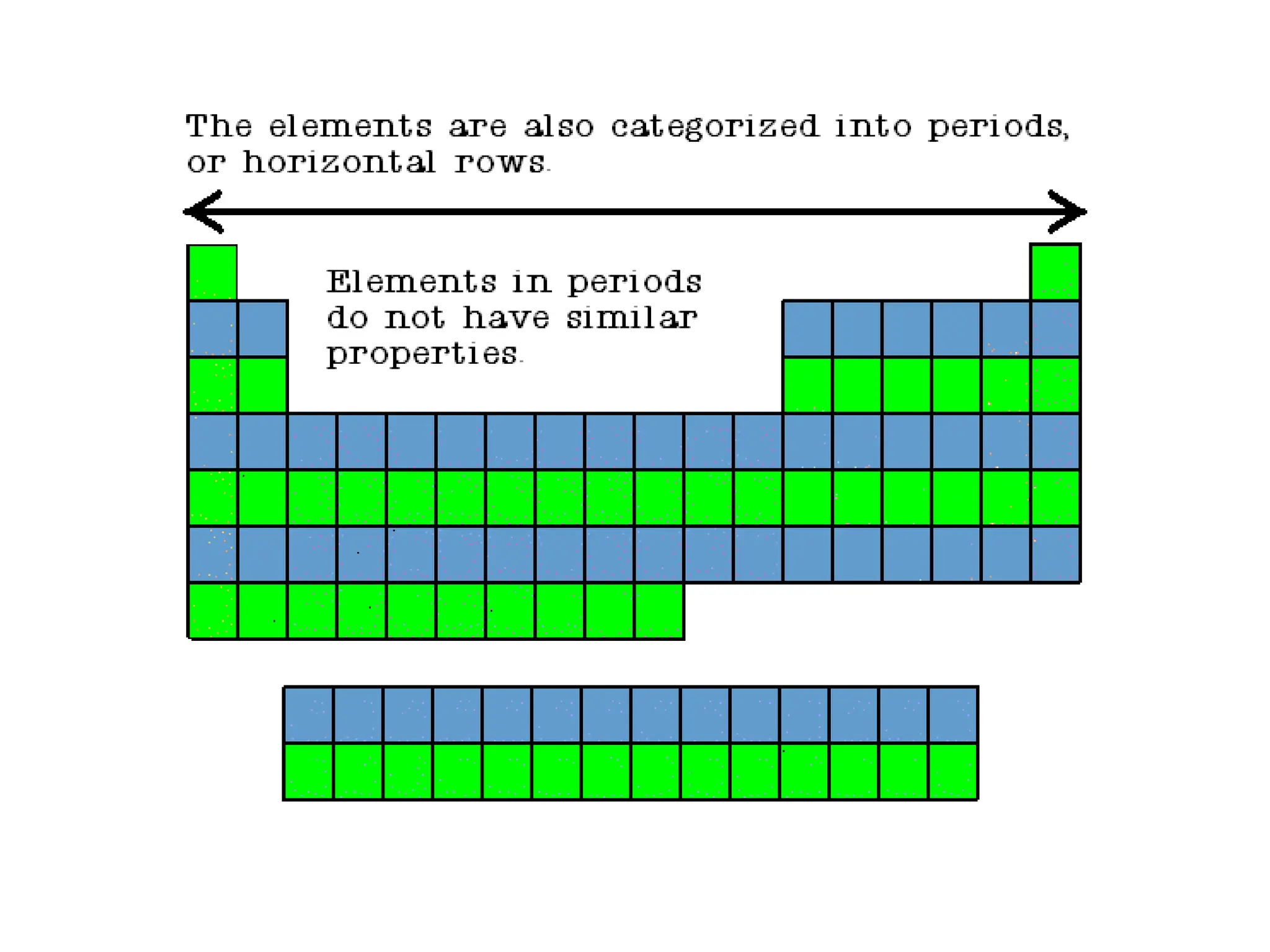
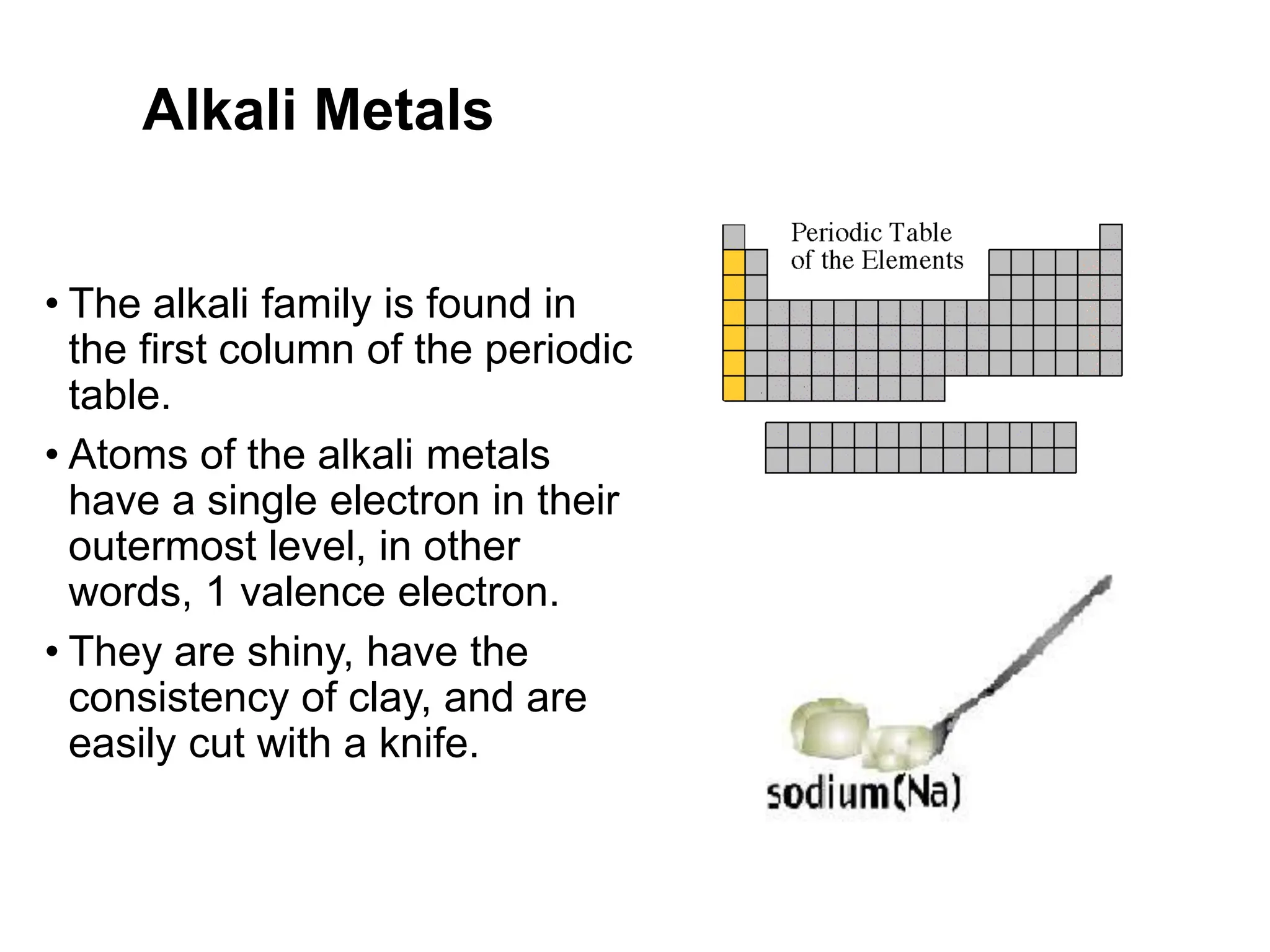
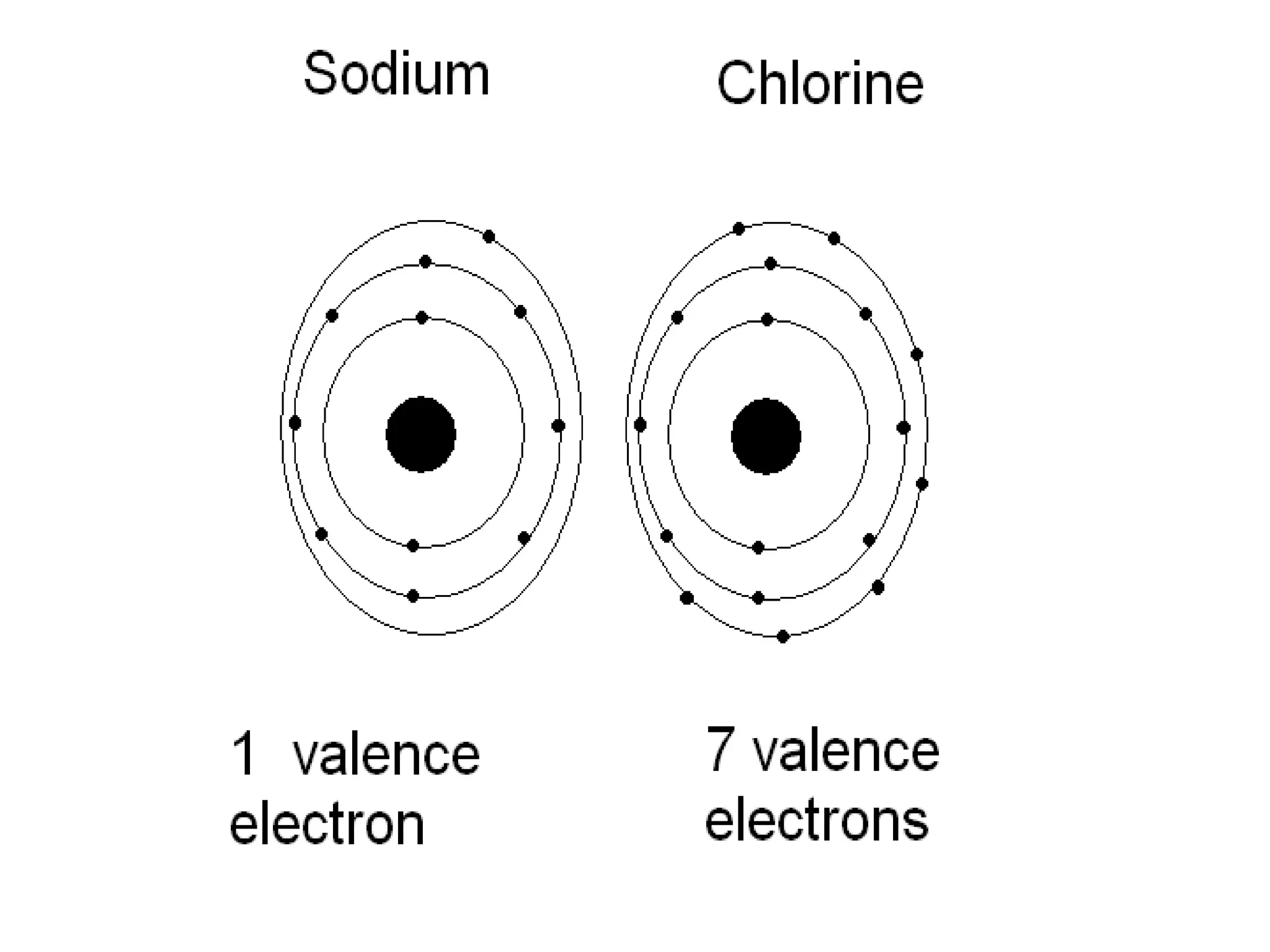
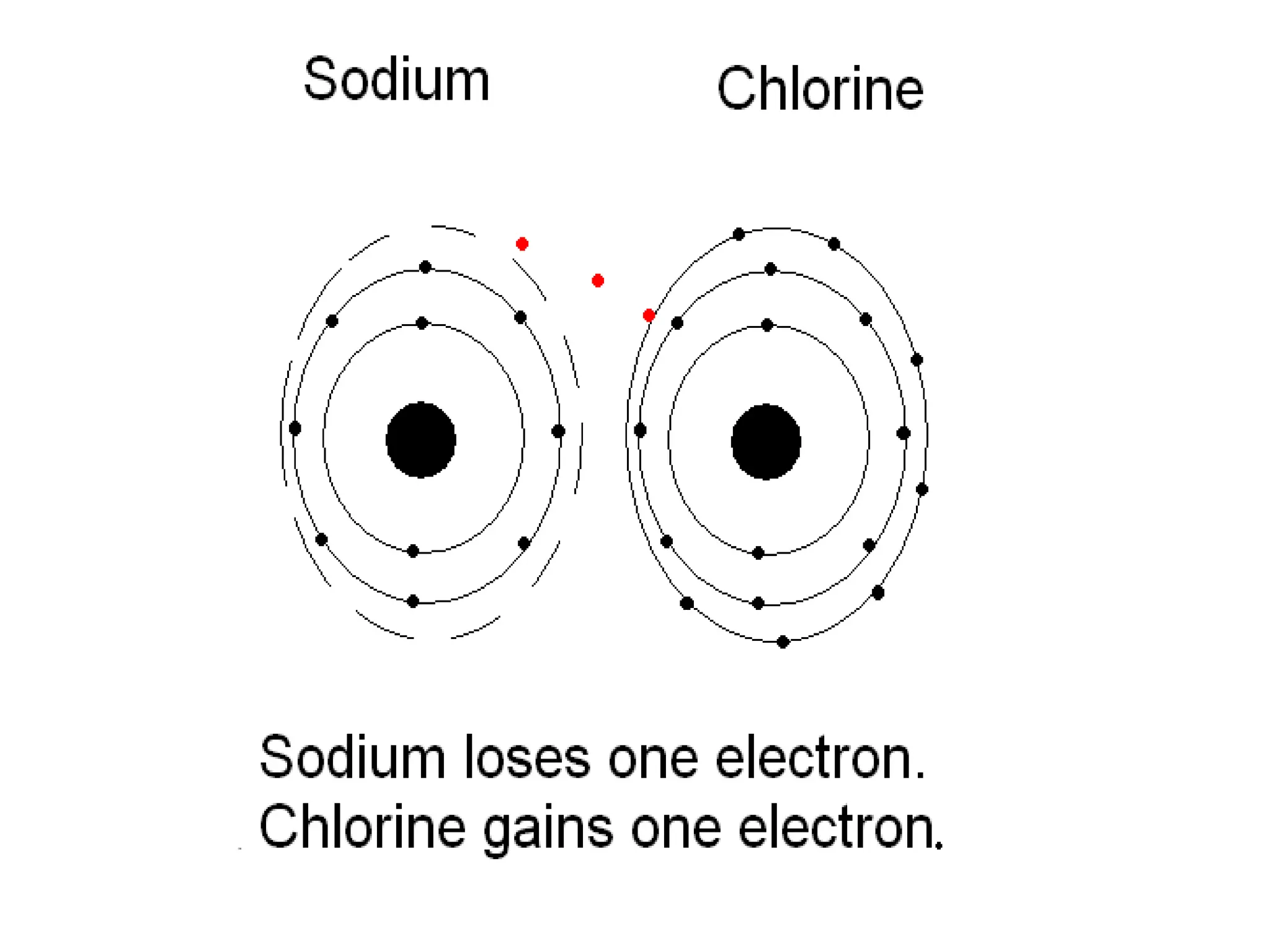
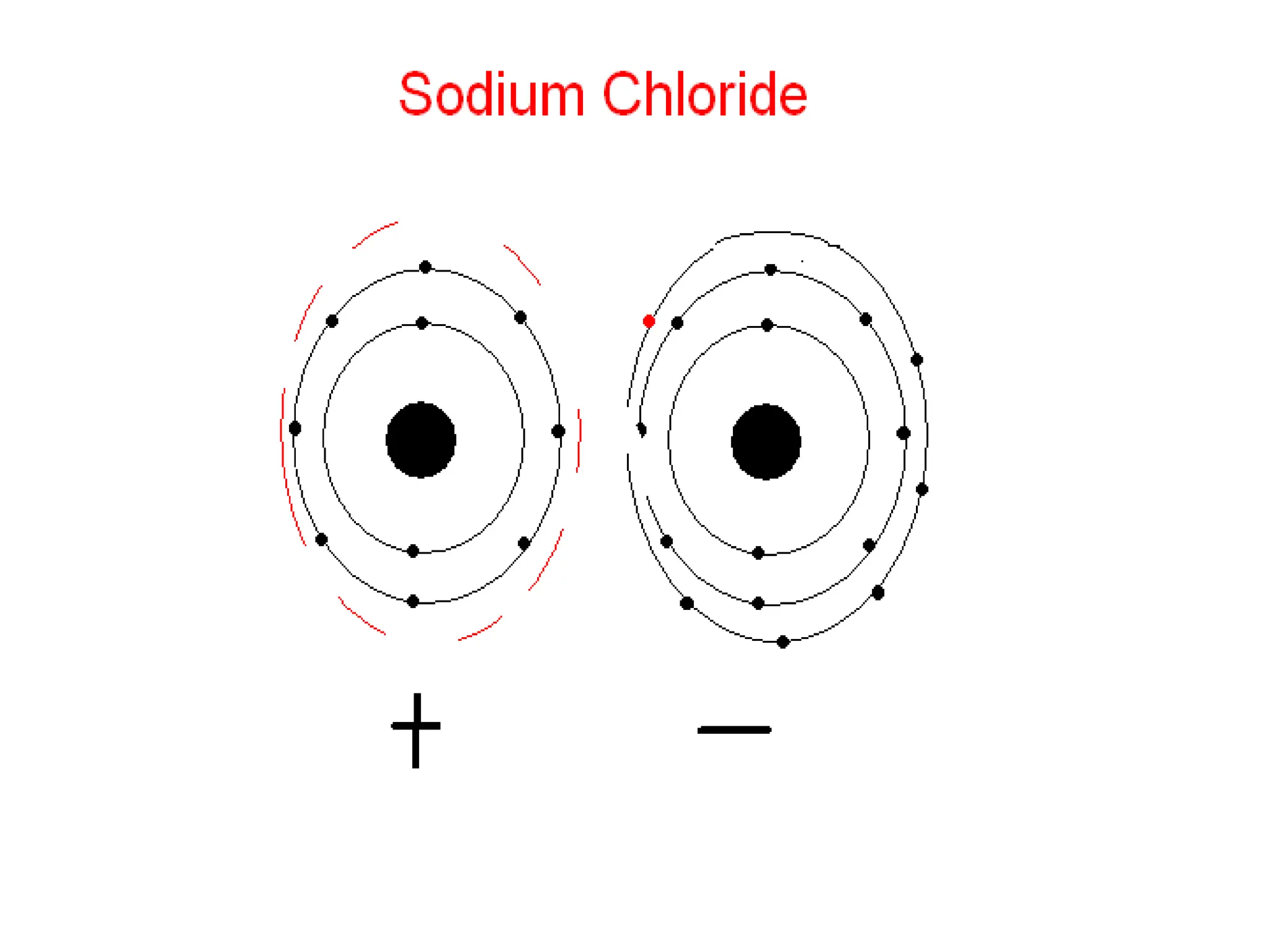
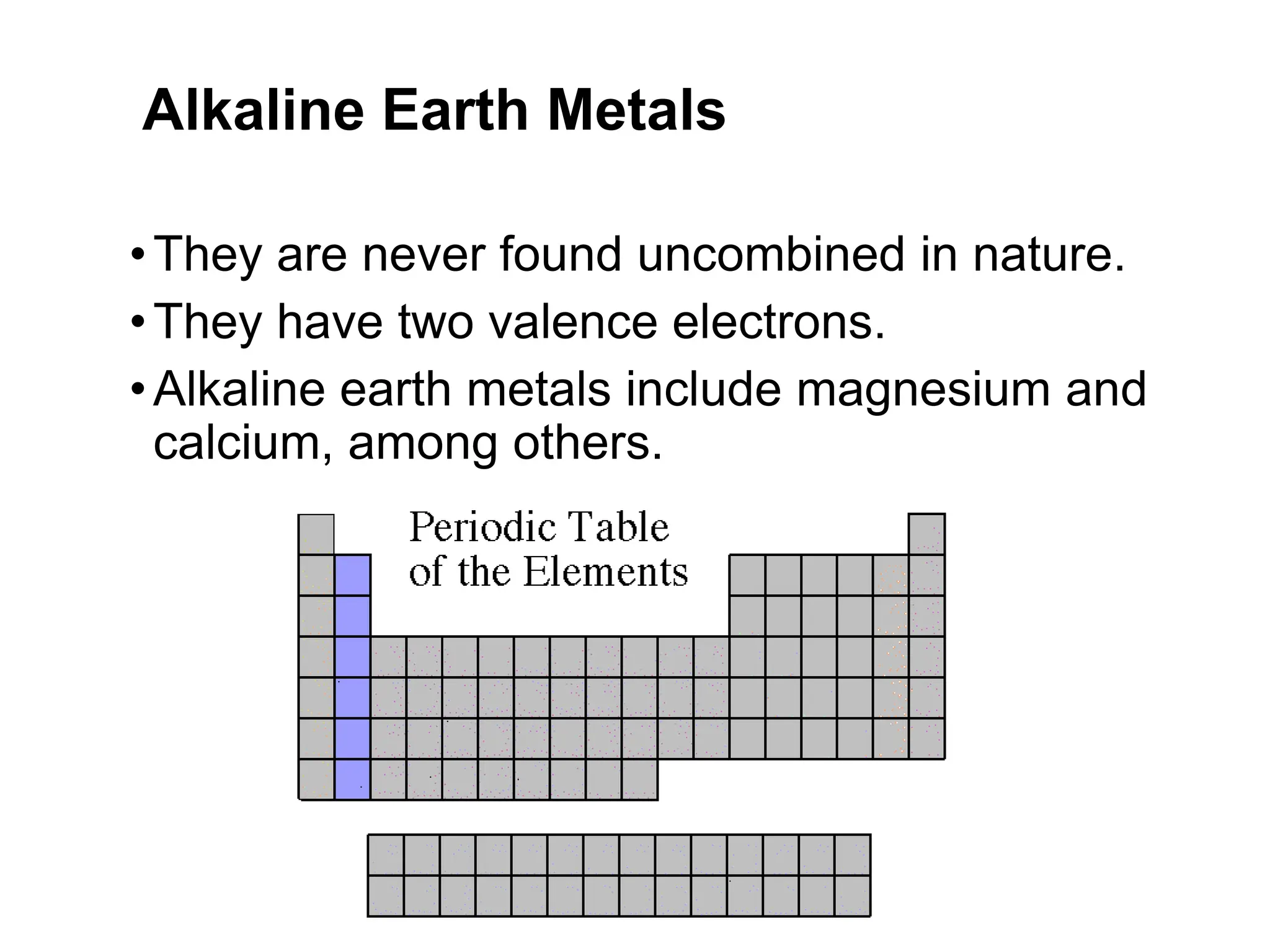
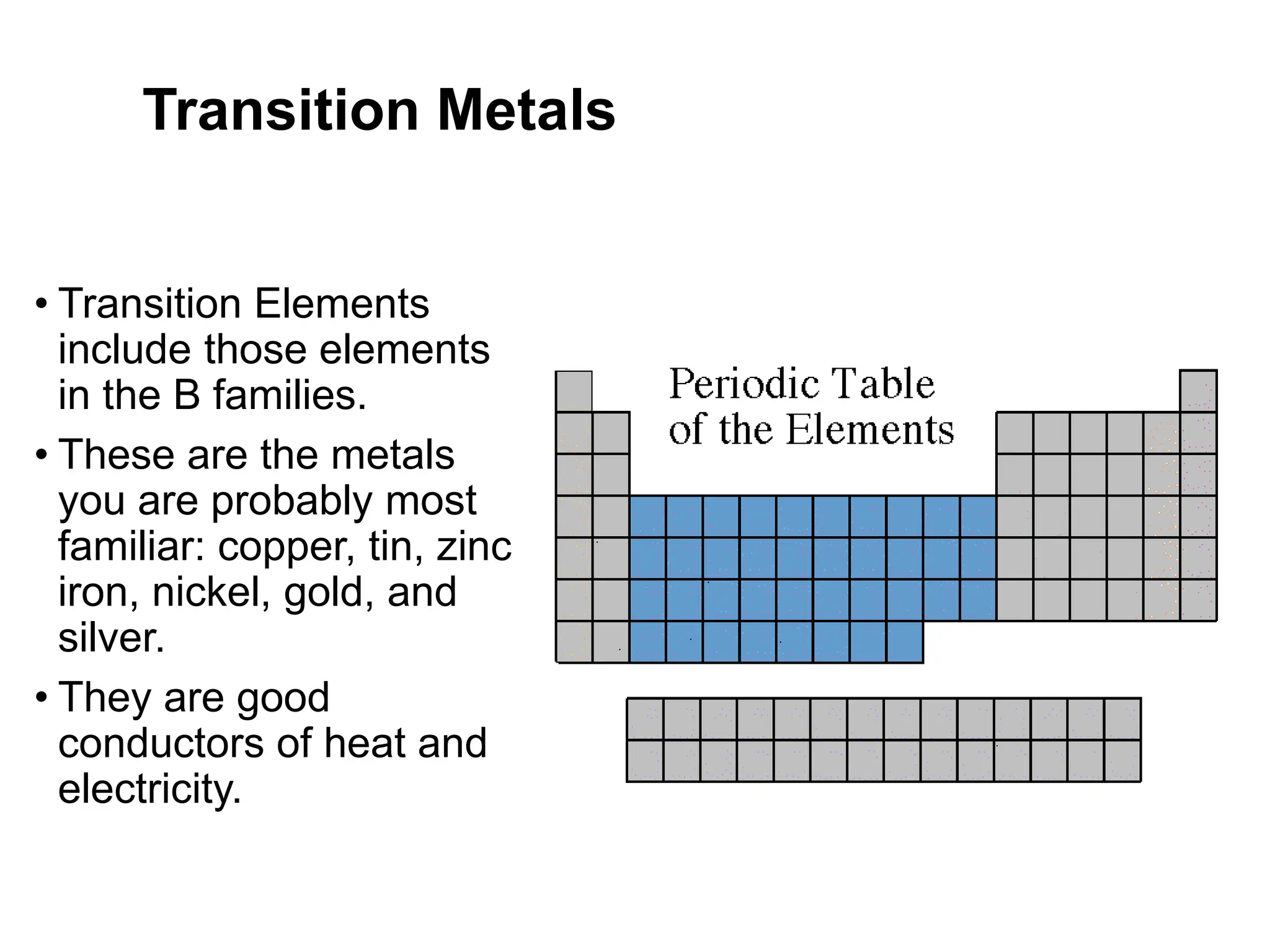
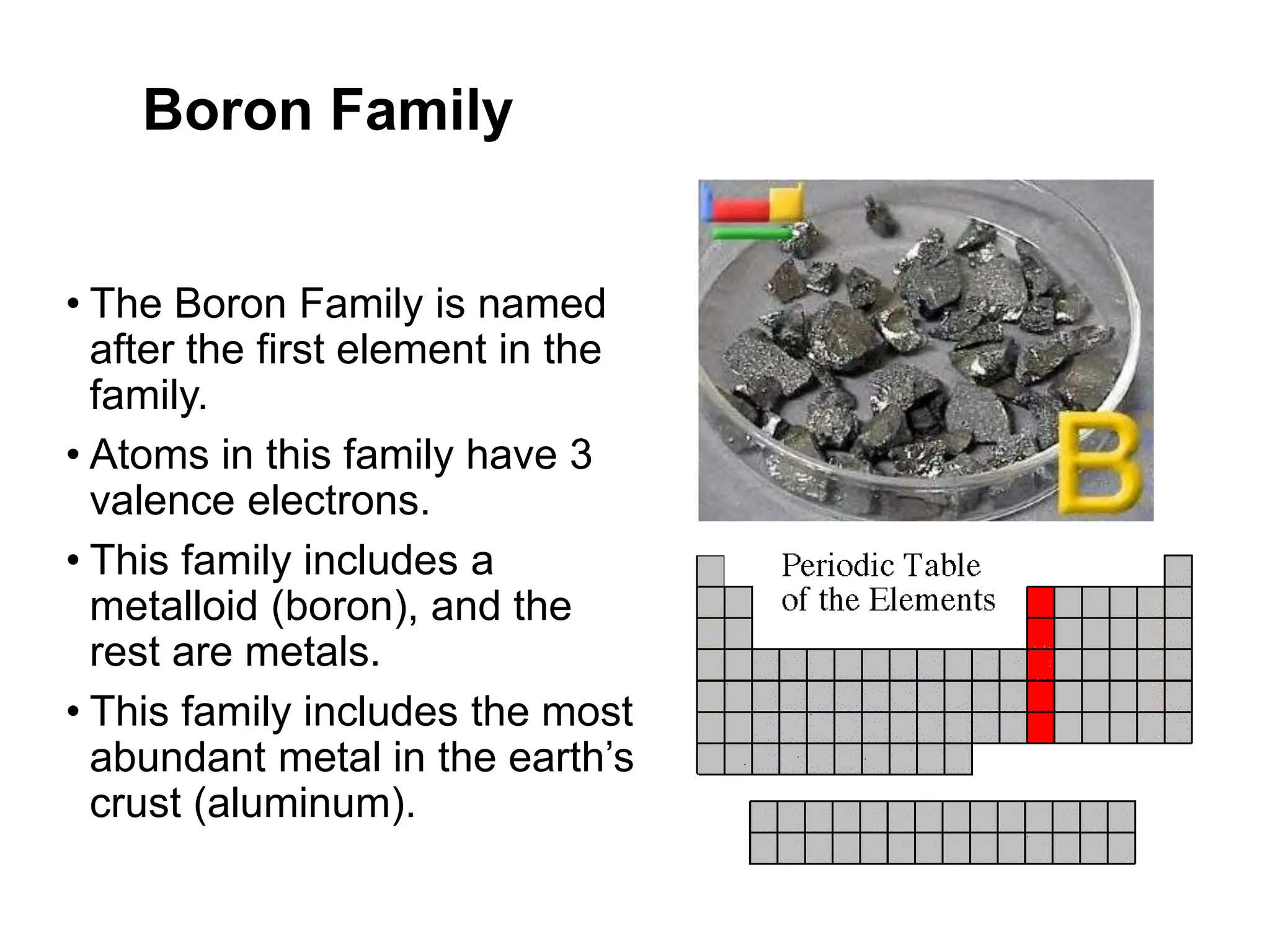
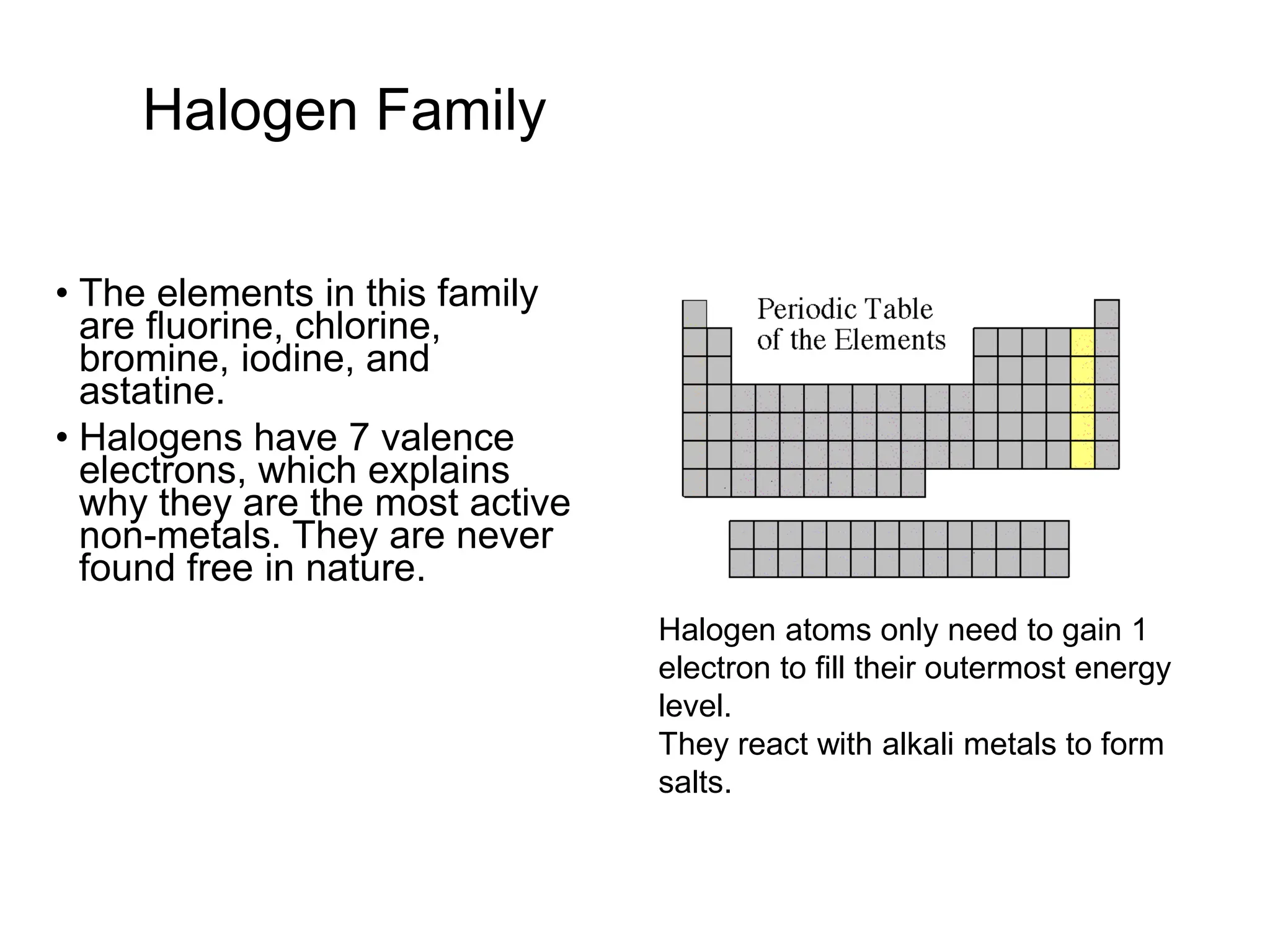
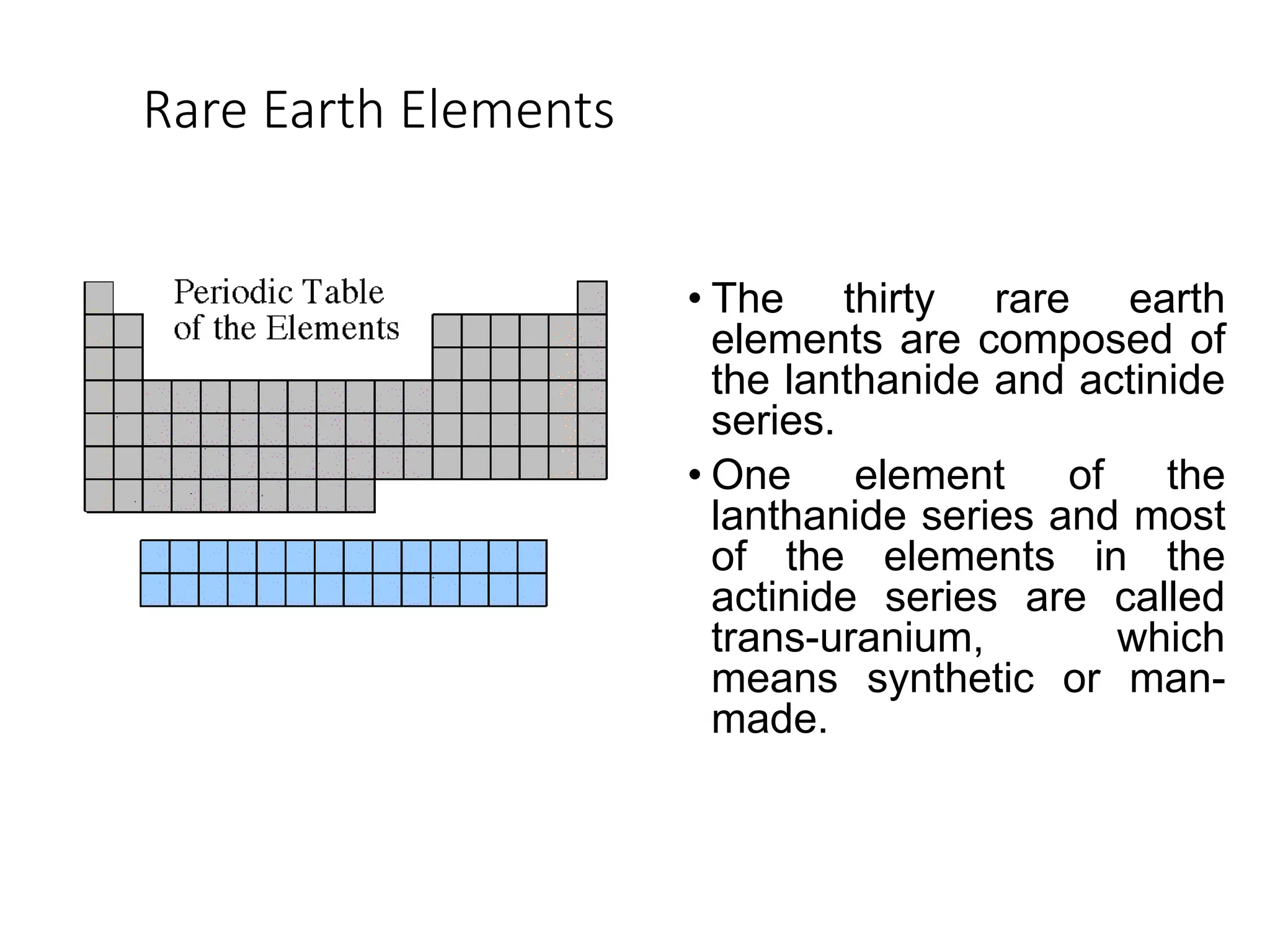
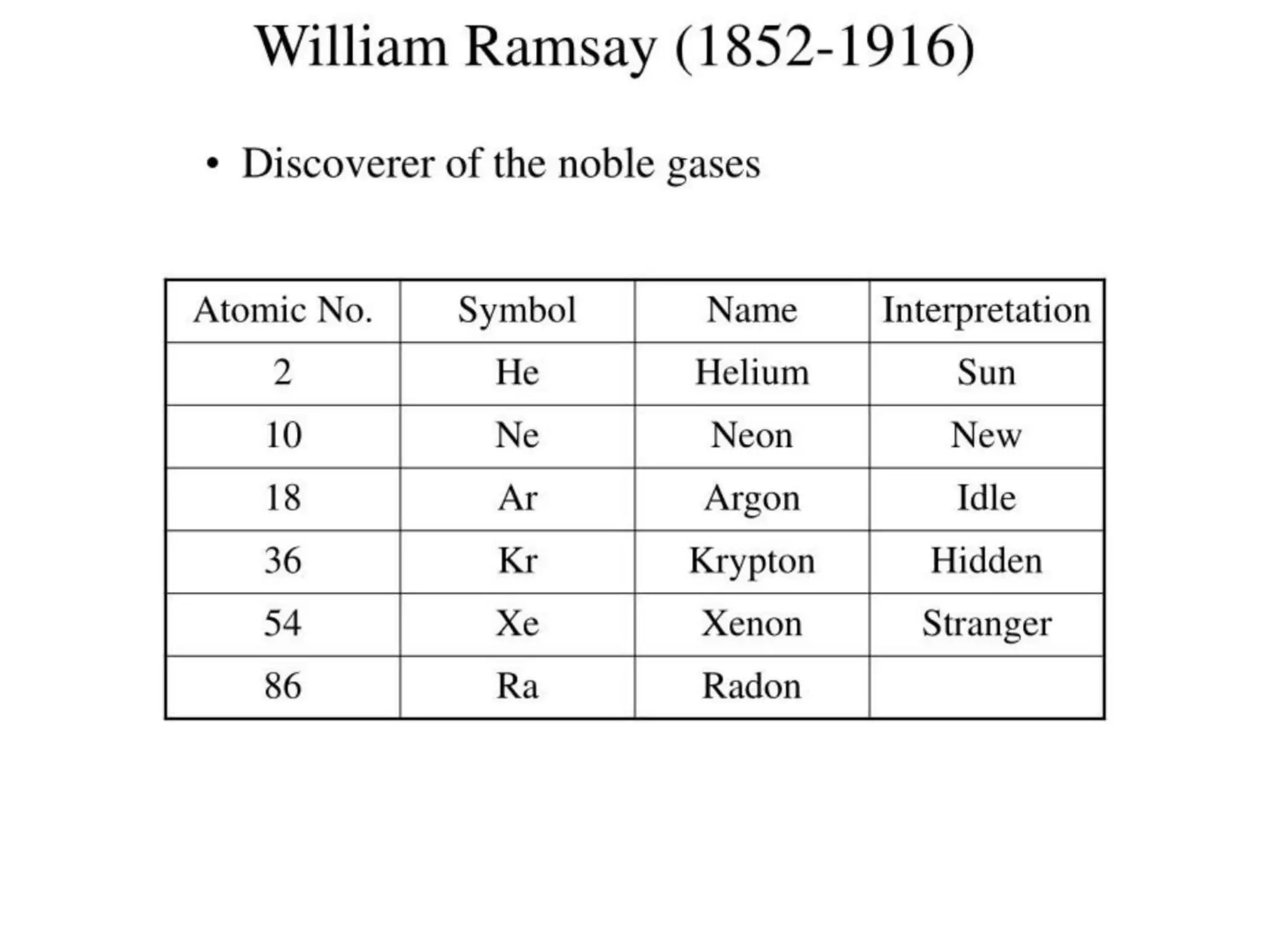
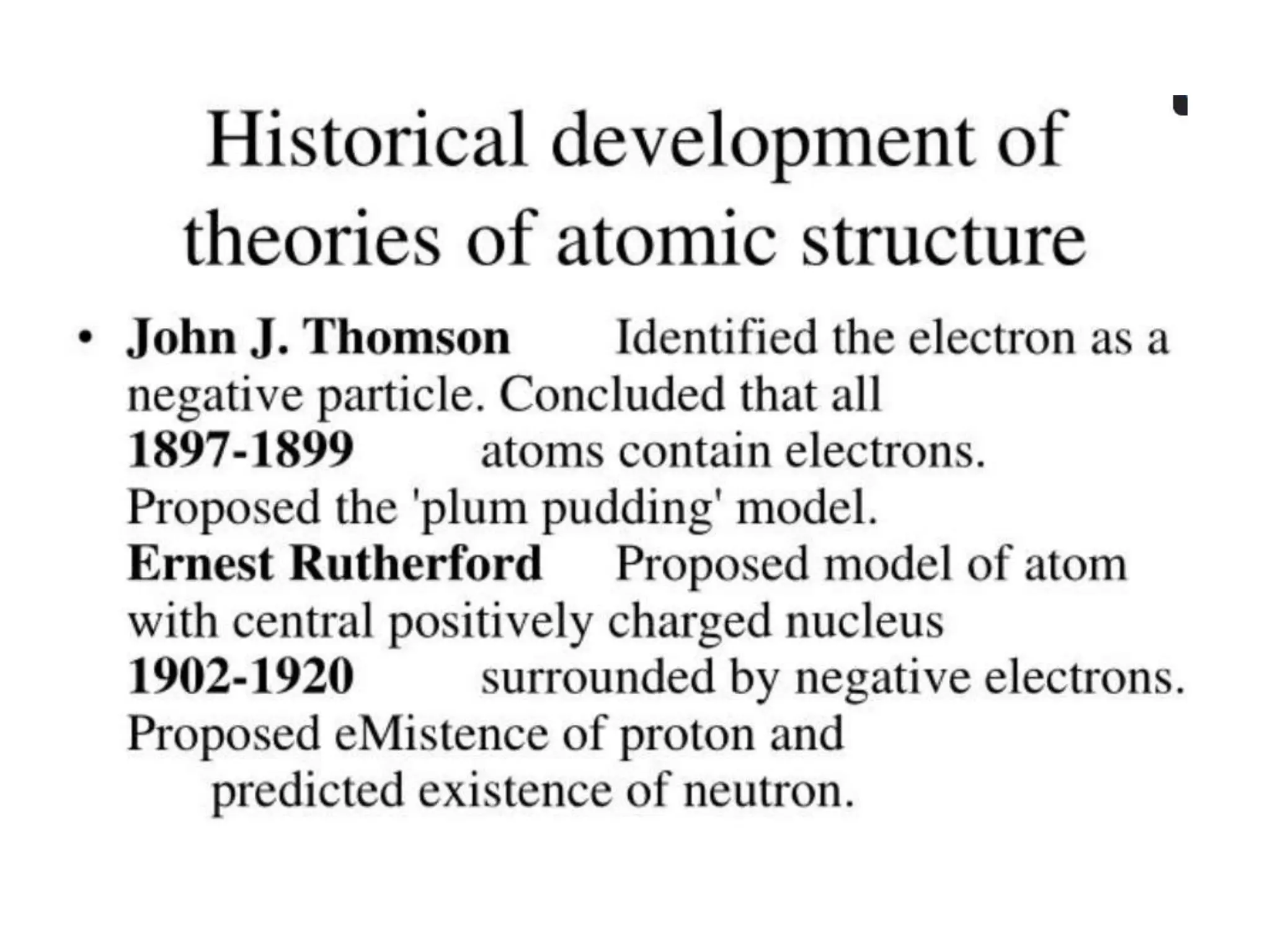
The document provides an overview of the periodic table, discussing the history and properties of various element groups such as alkali metals, alkaline earth metals, transition metals, and more. It highlights trends such as effective nuclear charge, atomic radii, ionization enthalpy, and electron gain enthalpy, alongside the characteristic behaviors of elements in each group. Additionally, it details the contributions of Dmitri Mendeleev in creating the first accepted periodic table in 1869.