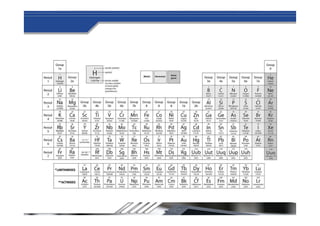
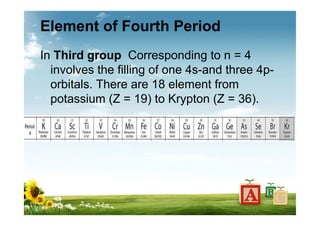
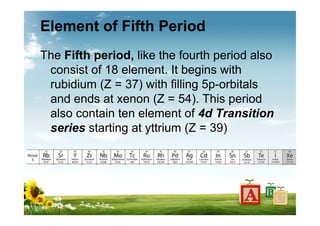
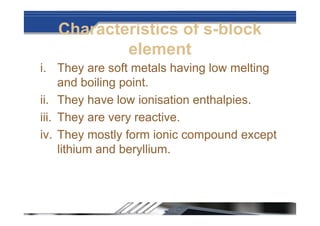
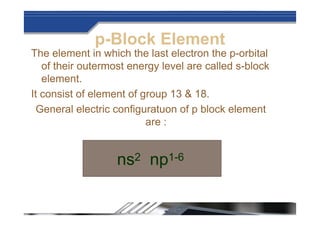
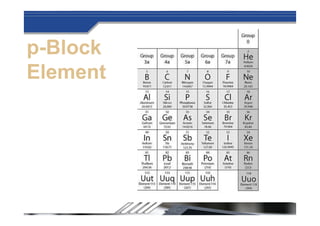
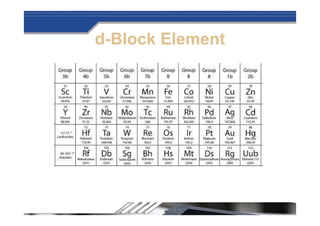
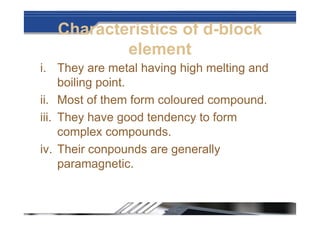
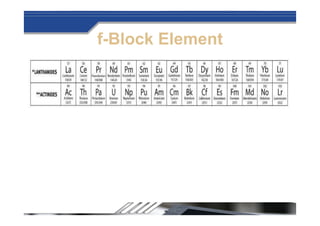
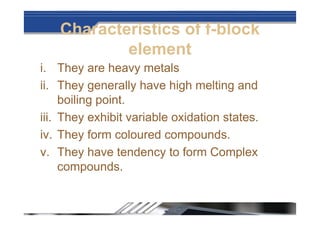
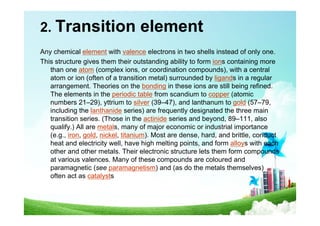
The document provides biographical information about Dmitri Mendeleyev, the discoverer of the periodic table. It notes that he was a Russian chemist born in 1834 who served as a professor of chemistry. In 1869, he announced the principle of periodicity of properties in chemical elements and created the first periodic table. His table arranged elements in order of atomic weight and grouped them by similar properties, allowing him to predict properties of undiscovered elements. The periodic table organized the known chemical elements and reflected recurring trends in their properties based on atomic structure.