Embed presentation
Download as PDF, PPTX



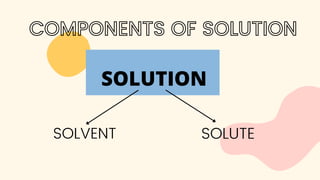
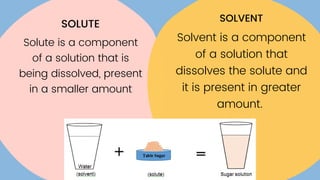


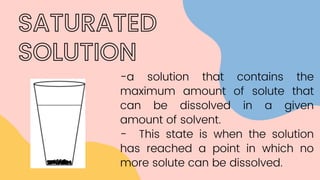
The document discusses saturated and unsaturated solutions. It defines a solution as a uniform mixture of a solute and solvent. A saturated solution contains the maximum amount of solute that can dissolve in a given amount of solvent, while an unsaturated solution contains less than the maximum amount of solute, meaning more can still dissolve. It explains that the solubility of a substance determines whether it will dissolve in a solvent to form a solution.







