The document discusses several gas laws:
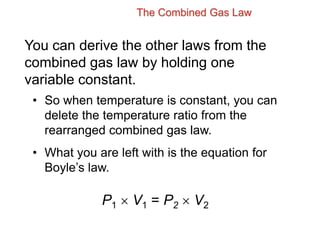
- Boyle's law states that for a fixed amount of gas at constant temperature, the volume is inversely proportional to the pressure.
- Charles's law describes the direct relationship between volume and temperature for a fixed amount of gas at constant pressure.
- Gay-Lussac's law explains that pressure and temperature are directly proportional for a fixed volume of gas.
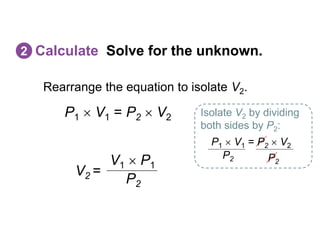
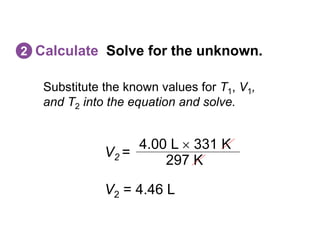
- The combined gas law incorporates all three by relating pressure, volume, and temperature for a fixed amount of gas. It can be used to derive the other gas laws by holding one variable constant. Sample problems demonstrate applying the gas laws to calculate unknown properties.