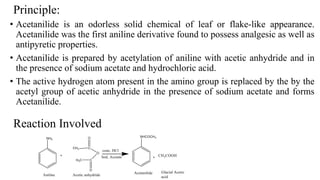
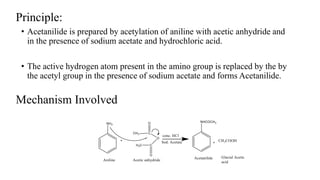
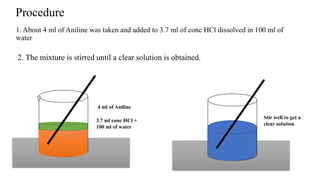
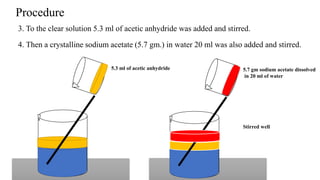
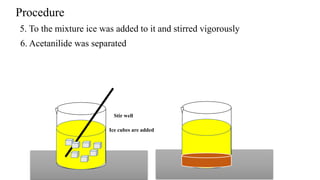
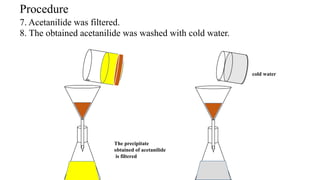
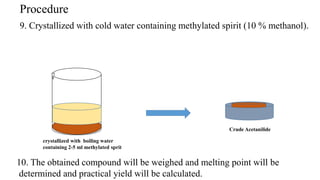
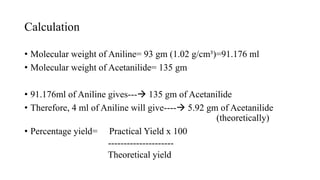
The document describes the procedure for preparing acetanilide from aniline. Specifically, it involves:
1) Dissolving aniline in hydrochloric acid and water to form a clear solution.
2) Adding acetic anhydride and sodium acetate to this solution, which results in the acetyl group replacing the active hydrogen in aniline's amino group to form acetanilide.
3) Precipitating the acetanilide product, filtering it, washing it with cold water, and crystallizing it with boiling water and methanol to obtain the final purified acetanilide. Yield is determined by comparing actual and theoretical amounts based on amounts of reactants used.