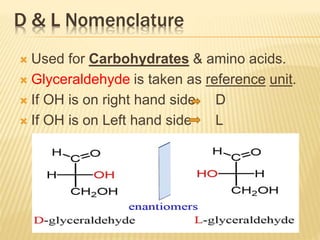
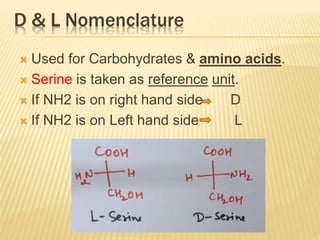
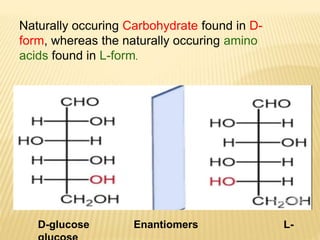
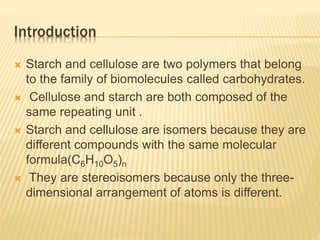
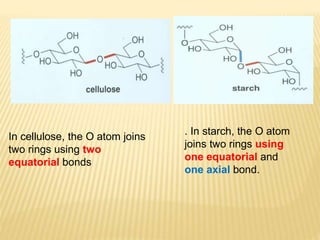
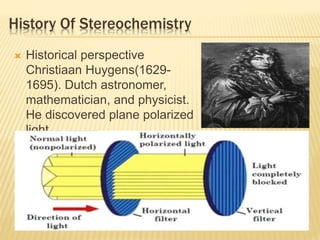
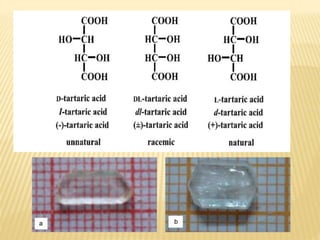
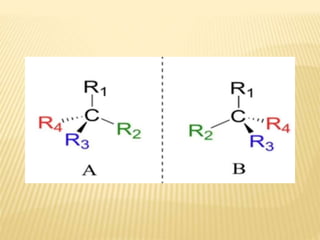
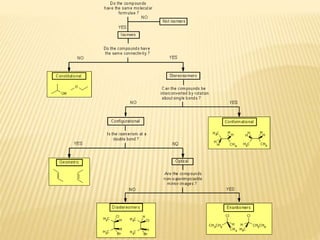
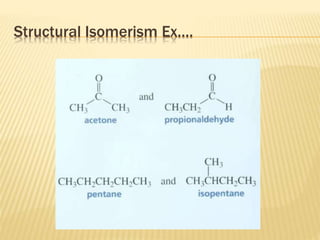
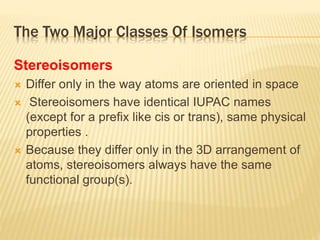
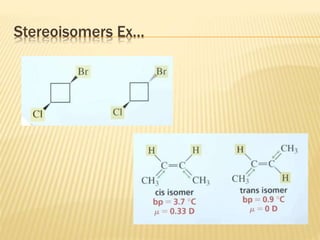
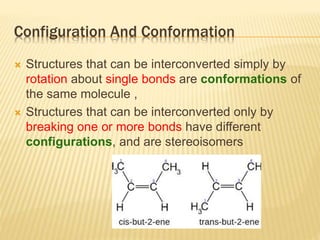
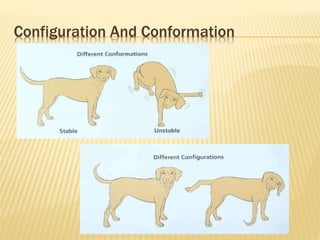
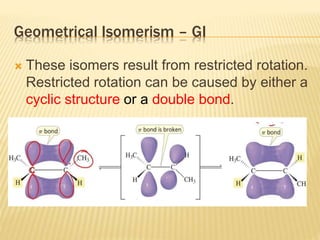
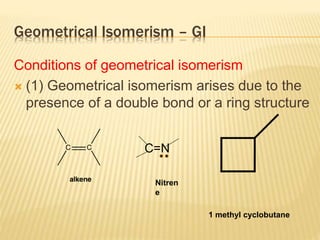
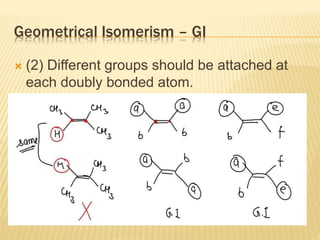
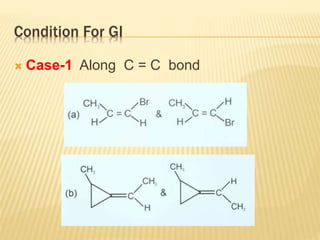
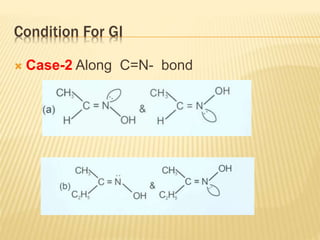
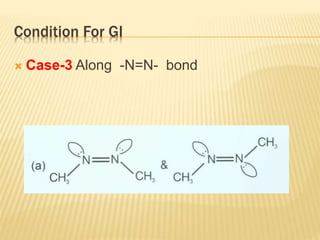
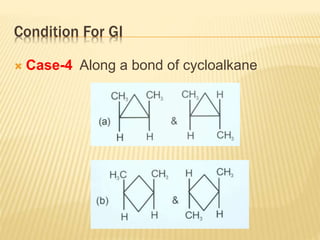
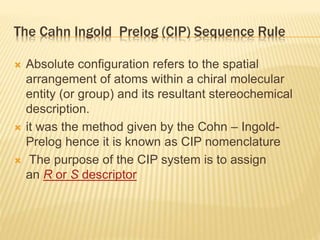
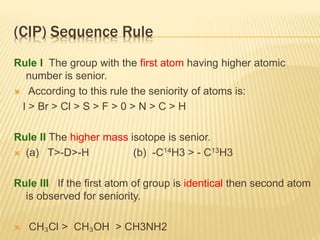
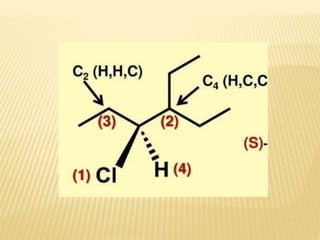
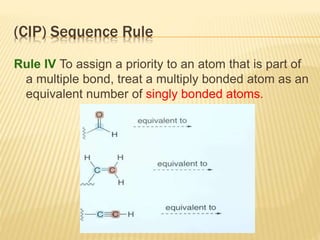
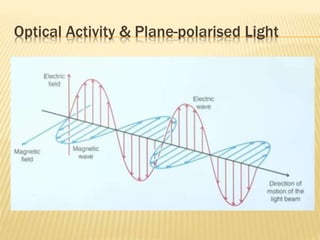
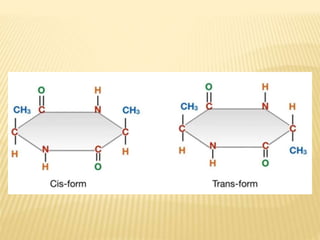
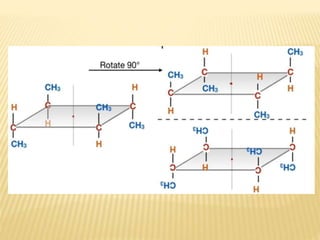
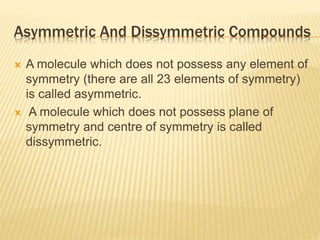
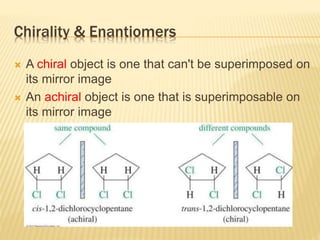
The document provides an extensive overview of stereochemistry and asymmetric synthesis, covering essential concepts such as optical activity, specific rotation, the Cahn-Ingold-Prelog sequence rule, and types of isomerism including meso compounds and geometrical isomerism. It discusses the historical development of stereochemistry and the significance of chirality, enantiomers, and methods of asymmetric synthesis. Additionally, it includes detailed explanations of nomenclature systems and the principles governing optical activity in organic compounds.






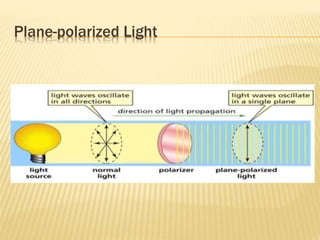
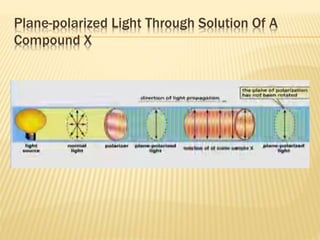
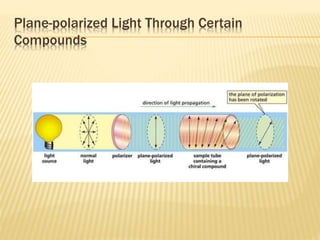

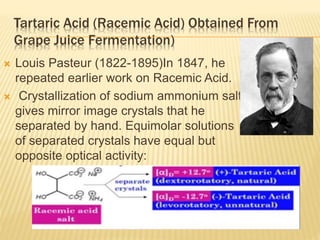








![Specific Rotation
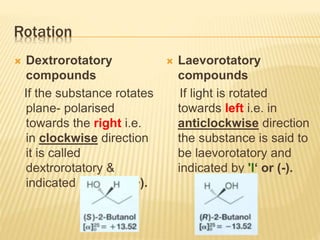
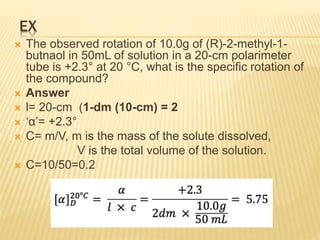
Specific rotation is the number of degrees of
rotation observed if a 1-dm (10-cm) tube is used
and the compound has concentration 1 gm/ml
[α ] = Specific rotation
θ =observed rotation (degree)
l = Pathlength (dm)
C=concentration (gm/ml)
λ=wavelength (nm)
t=temperature (25°C)
or](https://image.slidesharecdn.com/stereochemistryasymmetricsynthesis-240512072357-6b942b00/85/Stereochemistry-Asymmetric-Synthesis-pptx-55-320.jpg)
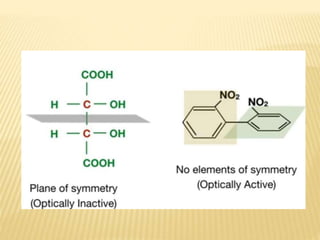

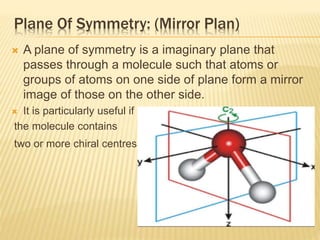
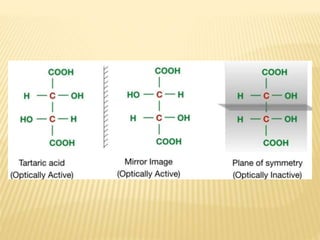
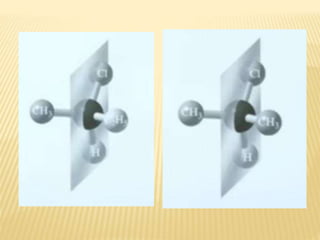
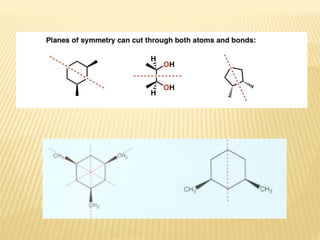
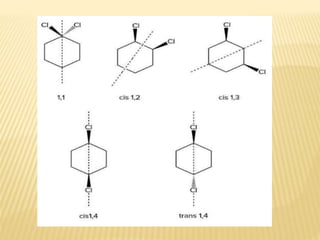
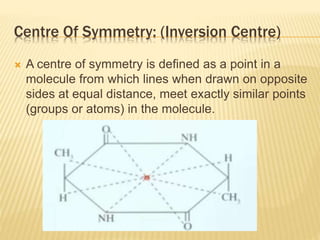
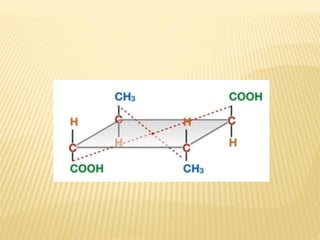



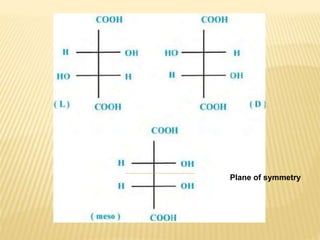
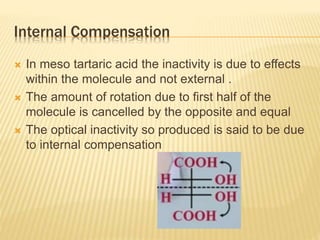
![Criteria For Meso Compund
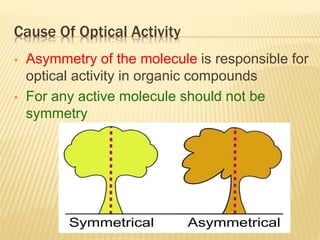
They are achiral (optical rotation = 0)
They have [a] = 0 due to internal
compensation of optical rotation
Presence of more than one asymmetric 'C'
atoms, no chiral center no meso .
They are non resolvable (optically inactive
compounds are non- resolvable).](https://image.slidesharecdn.com/stereochemistryasymmetricsynthesis-240512072357-6b942b00/85/Stereochemistry-Asymmetric-Synthesis-pptx-75-320.jpg)