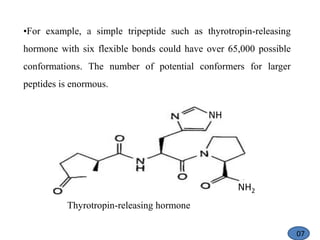
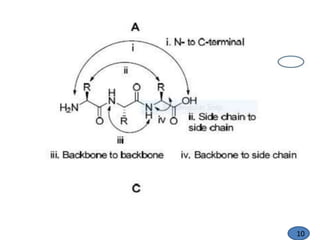
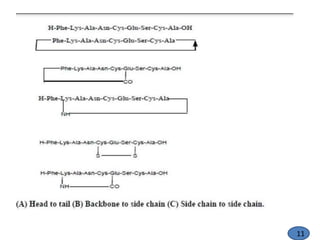
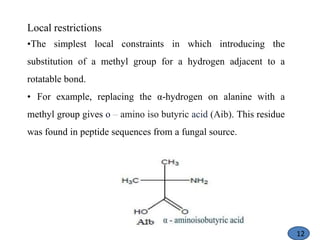
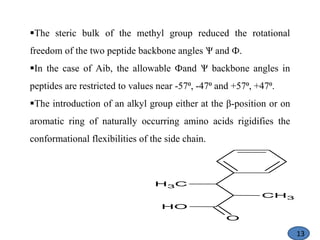
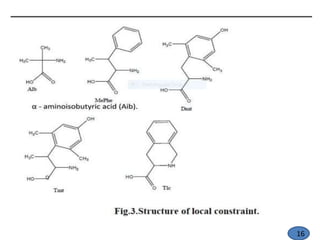
The document discusses modification of the peptide backbone and incorporating conformational constraints locally or globally to develop peptidomimetics. Peptidomimetics are designed to overcome stability issues with natural peptides and improve pharmacological properties. The peptide backbone can be modified through isoelectric or isoelectronic substitution or replacing the peptide bond with other chemical groups. Conformational constraints can be introduced globally through cyclization or locally by adding methyl groups or other alkyl substitutions to restrict backbone torsion angles. These modifications help reduce the number of accessible peptide conformations and increase selectivity, stability, and oral bioavailability.