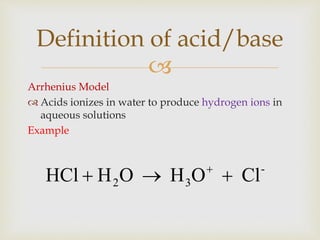
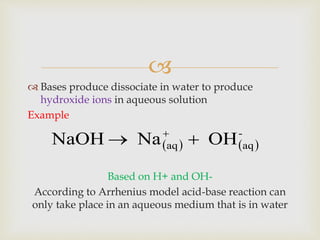
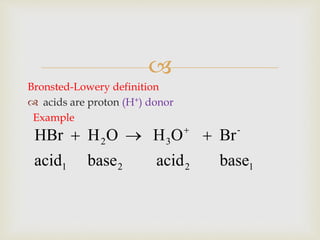
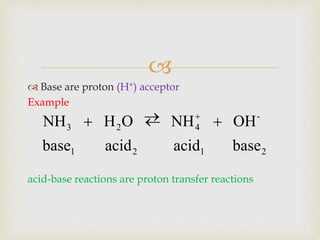
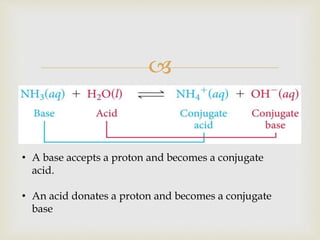
This document discusses different types of chemical reactions and properties of acids and bases. It describes four main reaction types - precipitation, acid-base, gas forming, and oxidation-reduction. It then explains properties of acids and bases according to the Arrhenius and Bronsted-Lowry theories of acids and bases. Key aspects covered are the Arrhenius definition of acids and bases producing H+ and OH- ions in water, and the Bronsted-Lowry definition of acids being proton donors and bases being proton acceptors. The concept of conjugate acid-base pairs is also introduced.