This document discusses acids and bases according to several theories:
- According to the Arrhenius theory, acids donate H+ ions and bases donate OH- ions in water. This theory does not explain behavior in non-aqueous solutions.
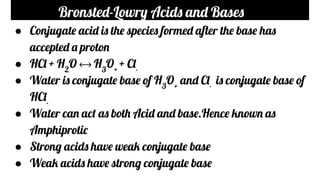
- The Bronsted-Lowry theory states that acids are proton donors and bases are proton acceptors. Conjugate acids and bases are formed in acid-base reactions.
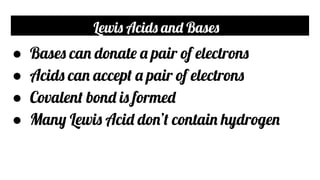
- The Lewis theory defines acids as electron pair acceptors and bases as electron pair donors. Covalent bonds are formed between them.
- Strong acids and bases fully dissociate in water while weak acids and bases only partially dissociate. Examples of strong and weak acids and bases are provided