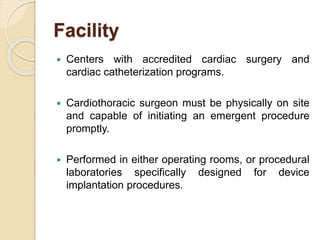
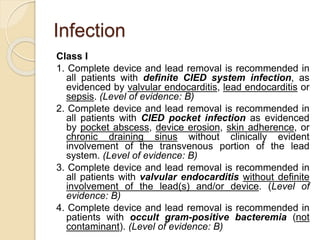
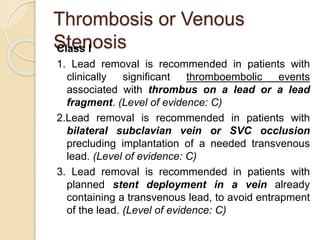
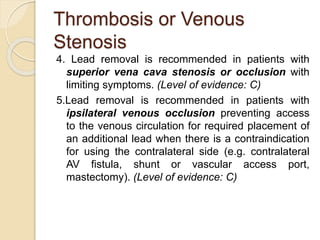
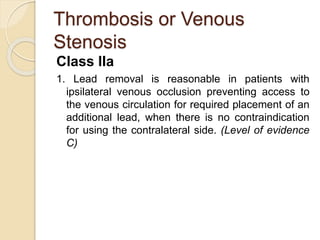
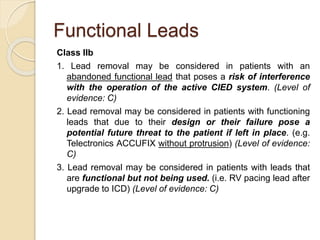
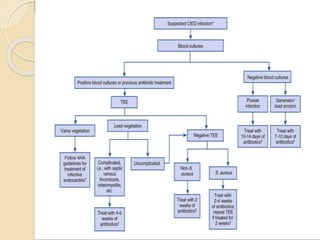
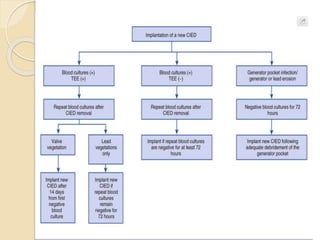
This document discusses transvenous lead extraction. It begins by providing background on the history of pacemakers and leads. It then defines various terms related to lead removal, extraction, and the different tools and techniques used. It discusses recommendations for operator training and facility requirements. Finally, it outlines the Heart Rhythm Society guidelines for indications for lead removal, including infection, pain, thrombosis, functional and non-functional leads. The guidelines classify recommendations as Class I, IIa, IIb or III based on evidence levels.



![ Heart Rhythm Society (HRS) [The North American
Society of Pacing and Electrophysiology,NASPE]
May 11, 1997, convened a policy conference to
formalize standards for:
training of physicians in extraction techniques;
equipment and emergently needed support staff at each
institution; and
indications and contra-indications for lead extraction.
Published as a guidance document in April 2000
and updated in 2009](https://image.slidesharecdn.com/transvenousleadextractionhrs2009-160920015225/85/Transvenous-lead-extraction-hrs-2009-7-320.jpg)



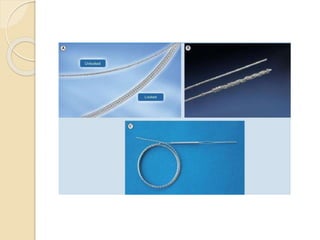
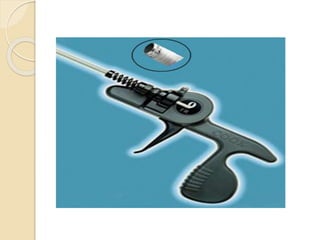
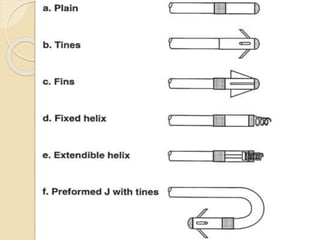
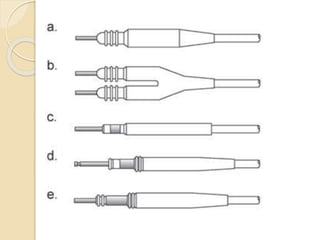
![Extraction tools
Simple Traction:
Manipulation of the lead so that the lead exits the
vasculature via the implant vein using tools
typically supplied for lead implant, with the addition
of traction [standard stylets (nonlocking), and
fixation screw retraction clips].
Traction Devices:
Specialized locking stylets, snares, sutures,
grasping or other devices used to engage or entrap
and remove the lead or lead fragments.](https://image.slidesharecdn.com/transvenousleadextractionhrs2009-160920015225/85/Transvenous-lead-extraction-hrs-2009-11-320.jpg)