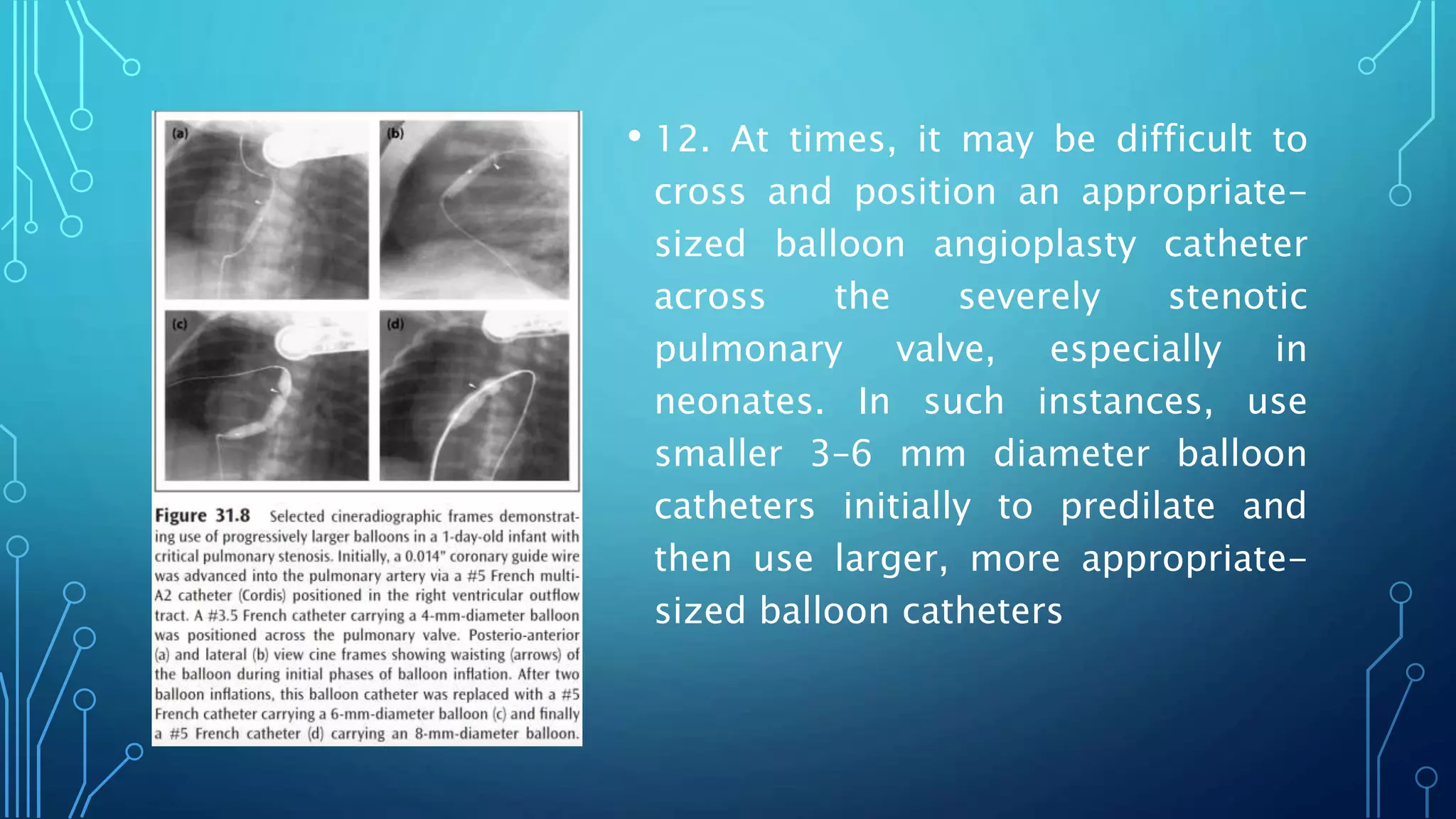
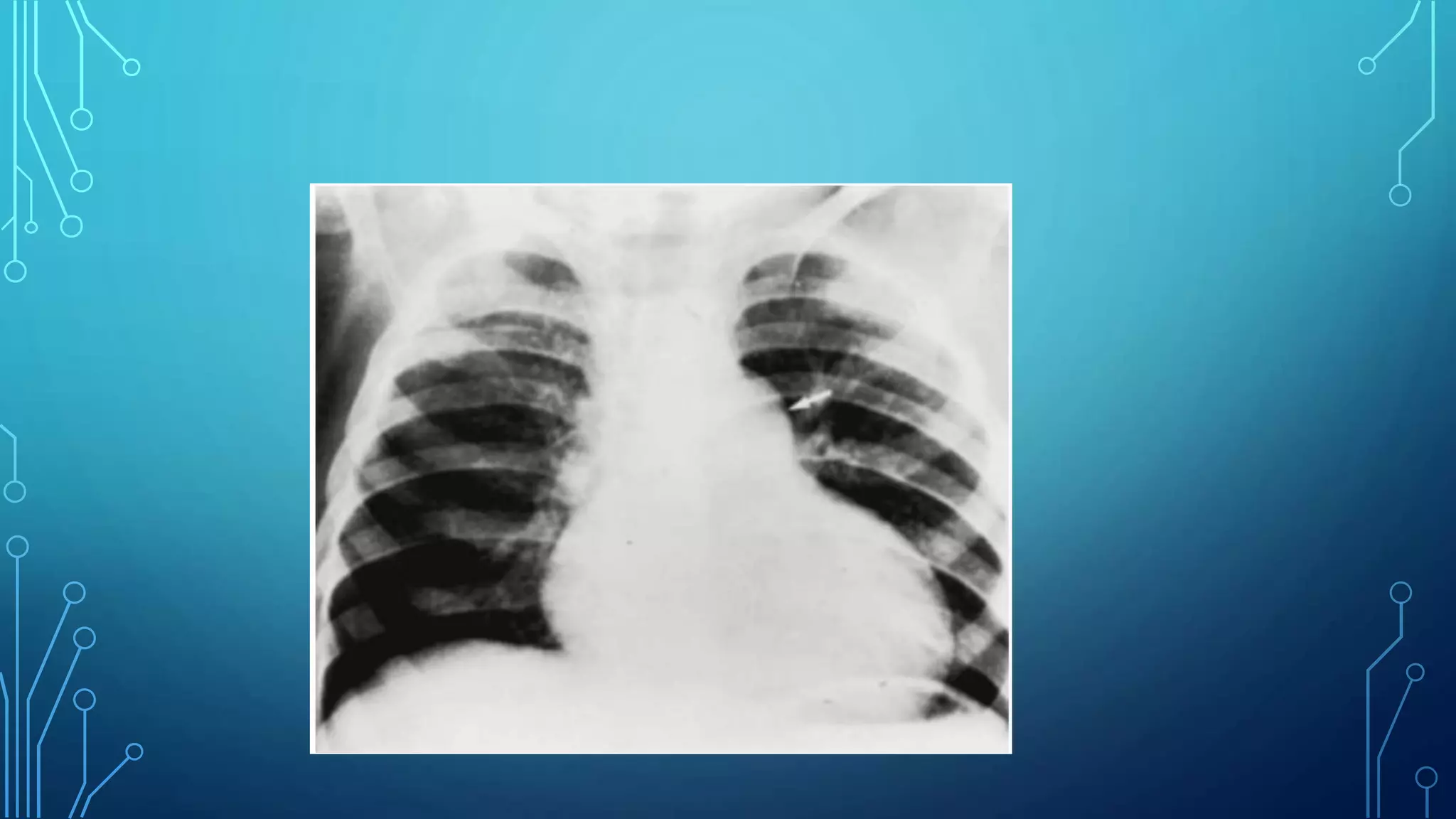
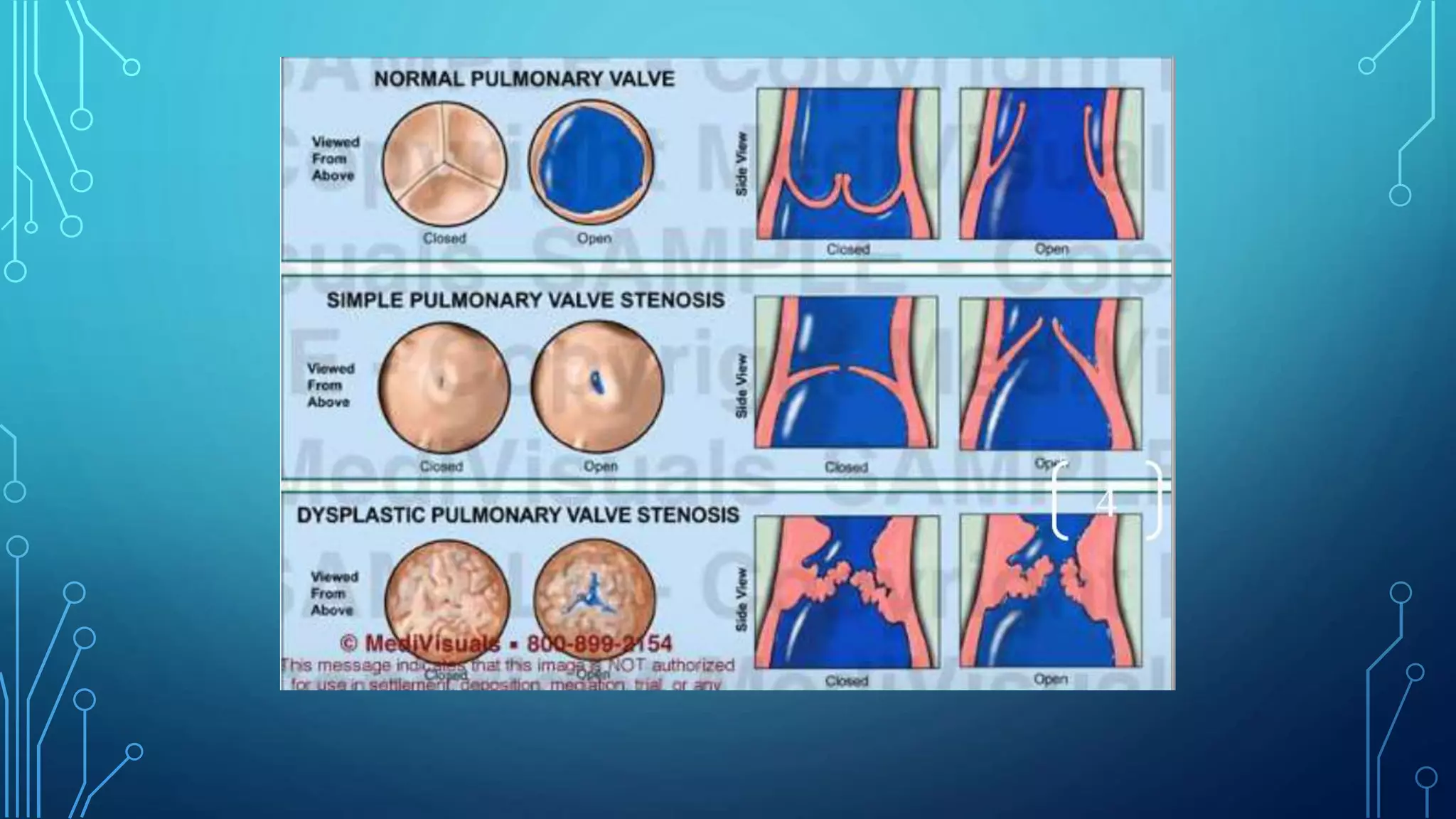
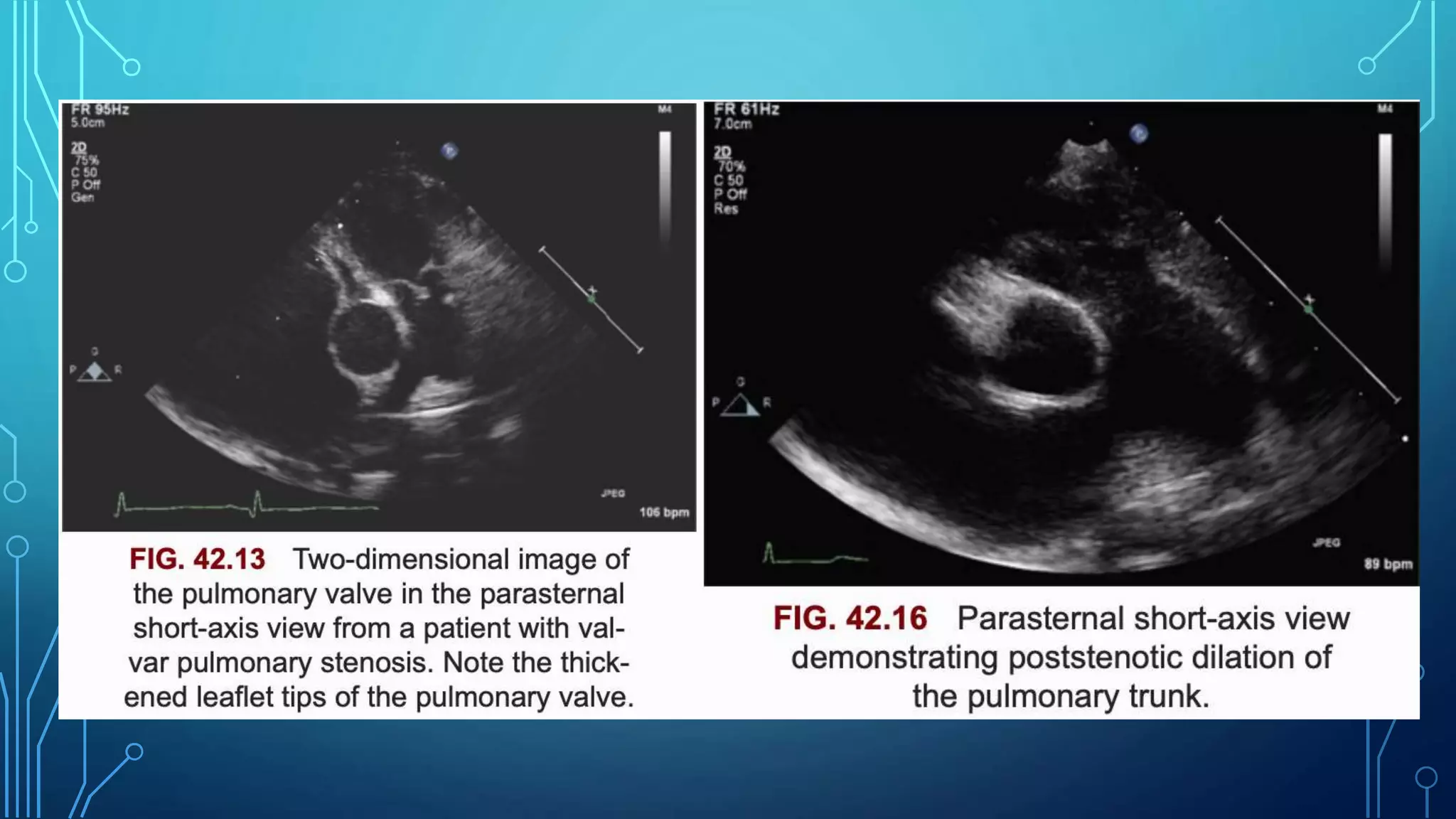
This document discusses pulmonary valve stenosis and balloon dilatation techniques. It provides background on the history and development of percutaneous pulmonary valvuloplasty. Key details include indications for the procedure, preprocedural evaluation and imaging, sedation and vascular access considerations, hemodynamic assessment, angiography, balloon catheter selection and use, and post-procedure protocol. The document serves as a reference for performing safe and effective balloon dilatation to treat pulmonary valve stenosis.





![INDICATIONS FOR BALLOON PULMONARY
VALVULOPLASTY
• Critical pulmonary stenosis, defined as pulmonary stenosis in a cyanotic
infant requiring a PDA to provide adequate pulmonary blood flow
• Resting catheterization peak systolic ejection gradient or echocardiographic
peak instantaneous pressure gradient 40 mm Hg or greater
• Resting catheterization or echocardiographic gradient less than 40 mm Hg
in the setting of RV dysfunction or symptoms
• Other indications for balloon pulmonary valvuloplasty, pulmonary atresia
with an intact ventricular septum but without RV-dependent coronary
circulation and as a palliative procedure for patients with cyanotic CHD
associated with pulmonary stenosis (e.g., tetralogy of Fallot [TOF]).](https://image.slidesharecdn.com/pvbd-200713142451/75/PVBD-8-2048.jpg)


























![9. A 4F to 6F multipurpose (multi A-2 [Cordis]) catheter is
introduced into the femoral venous sheath and advanced across
the pulmonary valve and the tip of the catheter is positioned in
the distal left (preferable) or right pulmonary artery. In neonates
and young infants, the catheter may be positioned in the
descending aorta via the ductus; this will increase the stability of
the wire and may make it easier to pass the balloon catheter
across the pulmonary valve](https://image.slidesharecdn.com/pvbd-200713142451/75/PVBD-41-2048.jpg)