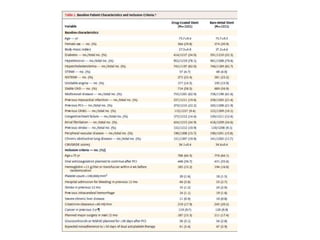
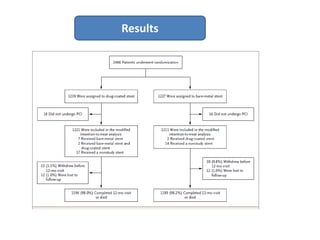
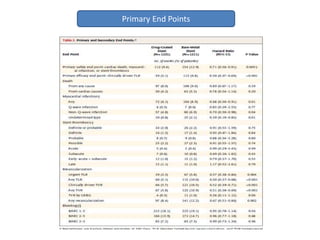
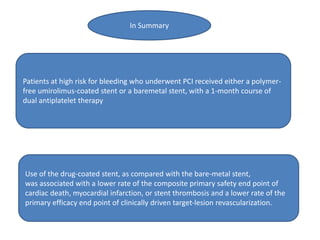
- The LEADERS FREE trial compared outcomes of patients at high risk of bleeding who received either a polymer-free drug-coated stent releasing umirolimus (BioFreedom stent) or a bare-metal stent, both with 1 month of dual antiplatelet therapy.
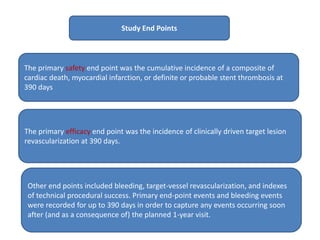
- Use of the drug-coated stent was associated with lower rates of the primary safety composite of cardiac death, MI, or stent thrombosis and the primary efficacy endpoint of clinically-driven TLR compared to the bare-metal stent.
- The drug-coated stent showed benefit over the bare-metal stent in reducing rates of MI, likely driven by lower rates of in-stent restenosis, without increasing the risk of bleeding which