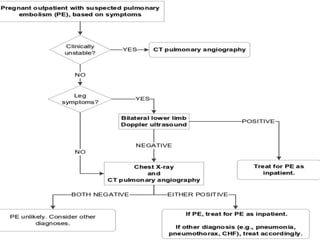
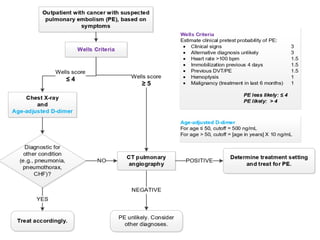
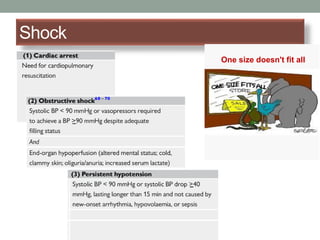
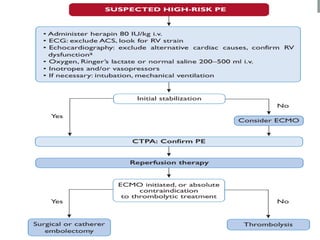
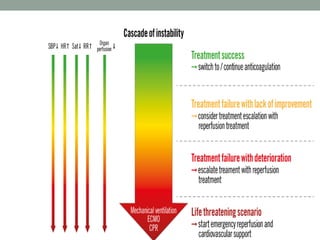
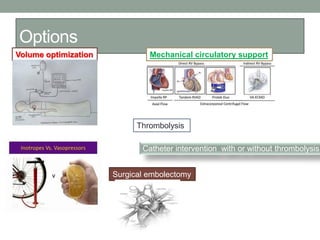
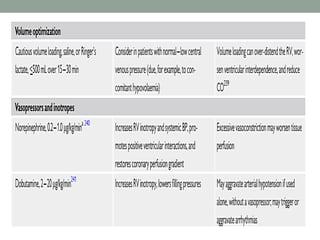
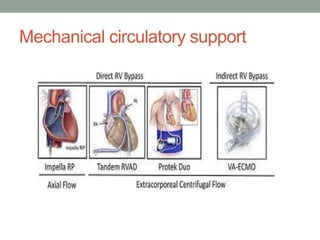
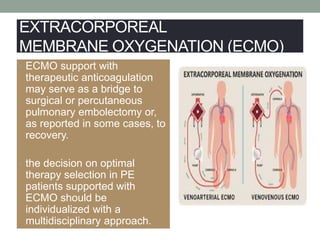
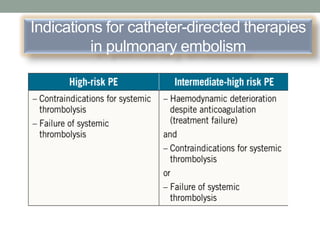
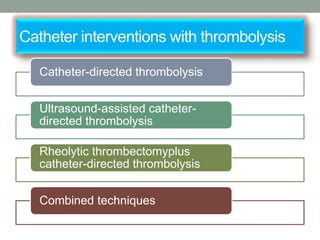
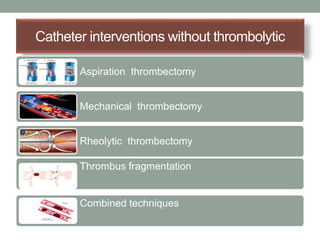
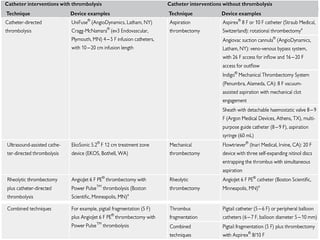
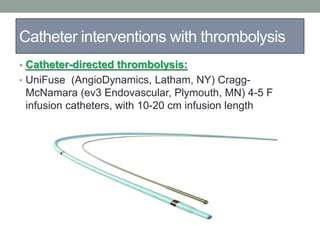
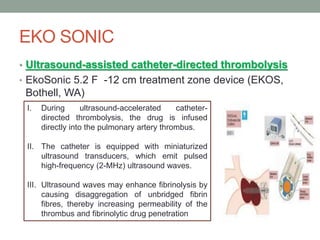
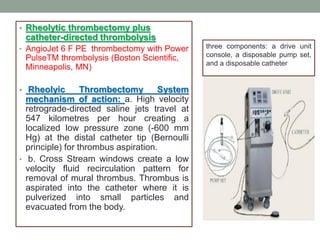
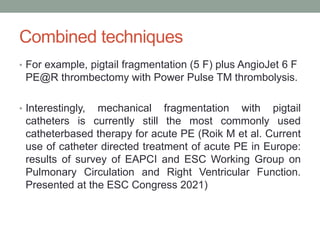
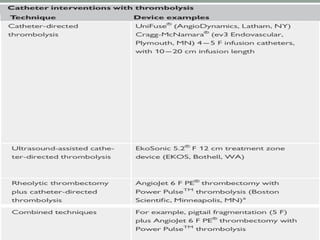
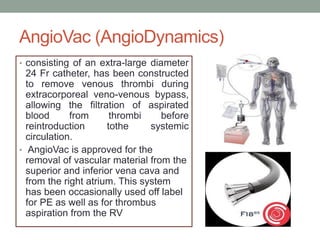
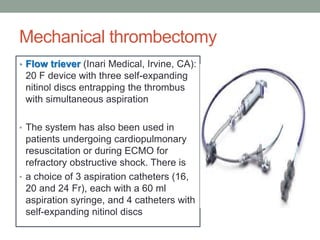
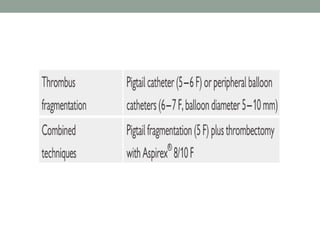
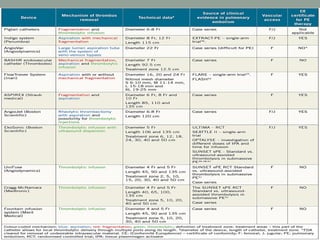
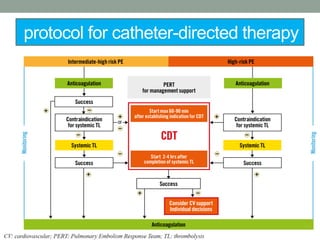
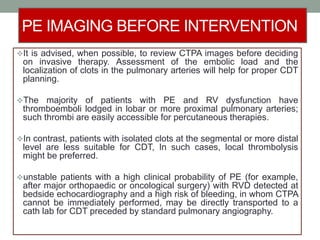
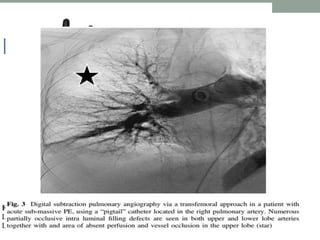
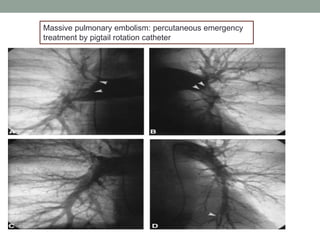
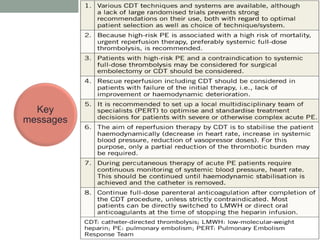
The document discusses various management strategies for pulmonary embolism (PE), emphasizing that treatment should be tailored to individual patient needs and local healthcare resources. It covers non-pharmacological interventions such as catheter-directed therapies, surgical embolectomy, and mechanical circulatory support options like ECMO and Impella RP, highlighting their indications and efficacy. Protocols for catheter-directed interventions include patient assessment, procedure steps, and post-treatment management to ensure optimal outcomes.