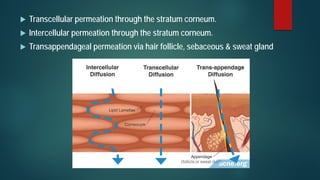
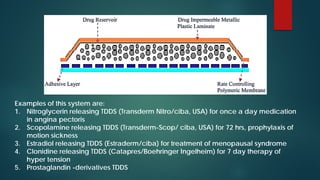
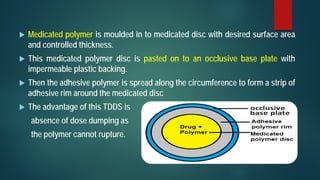
This document discusses transdermal drug delivery systems (TDDS). It begins by defining TDDS and listing some of its advantages and disadvantages. It then covers topics like permeation through skin, factors affecting permeation, permeation enhancers, and the basic components of a TDDS including polymers, drugs, pressure sensitive adhesives, and excipients. It discusses formulation approaches and various routes of drug penetration across skin like transcellular, intercellular, and transappendageal routes.