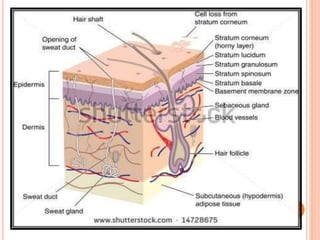
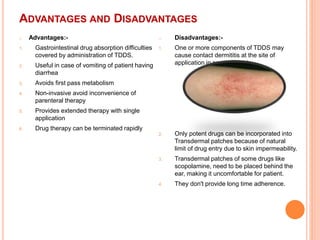
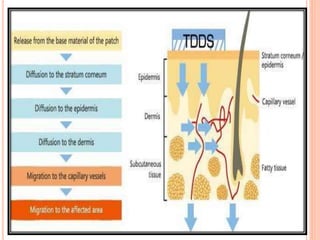
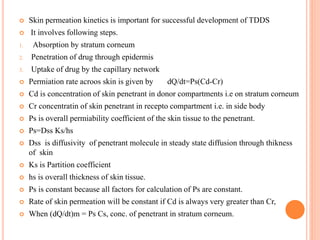
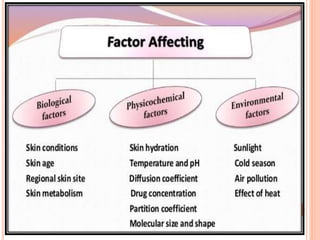
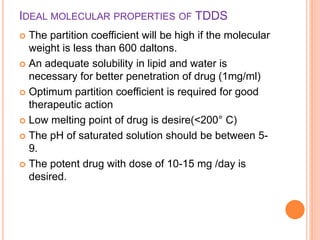
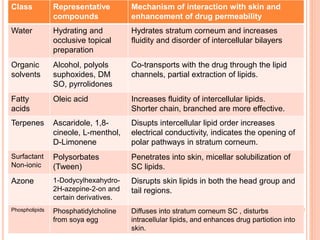
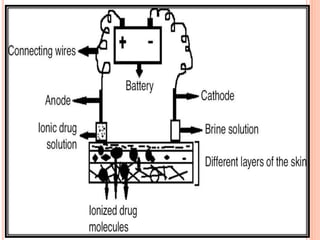
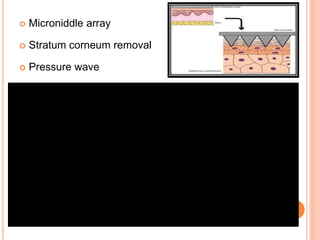
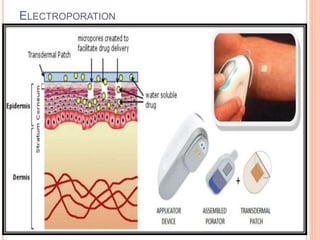
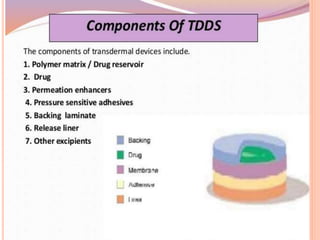
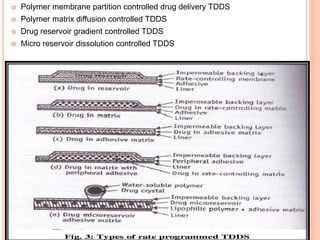
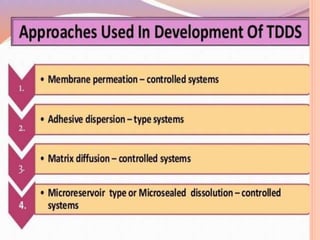
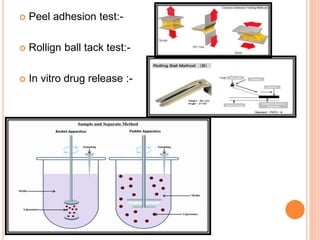
This document discusses transdermal drug delivery systems (TDDS). It outlines the advantages of TDDS, including avoiding gastrointestinal issues and first-pass metabolism. It also notes some disadvantages like skin impermeability limiting drug choice and potential irritation. The document explains the process of transdermal permeation and factors affecting it like skin hydration, temperature, and drug properties. It covers enhancement techniques to increase permeation like chemical enhancers, iontophoresis, electroporation, and microneedle arrays. Ideal drug properties for TDDS and evaluation methods are also summarized.
![TRANSDERMAL DRUG DELIVERY
SYSTEM
Prepared By :- Mugdha A Joshi]
Asst. Professor,
IVM’s, IIPER,
Talegaon Dabhade.](https://image.slidesharecdn.com/unitiiitransdermaldrugdeliverysystem-201013050714/75/Unit-iii-transdermal-drug-delivery-system-1-2048.jpg)