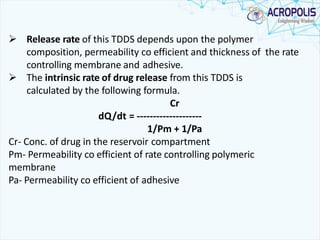
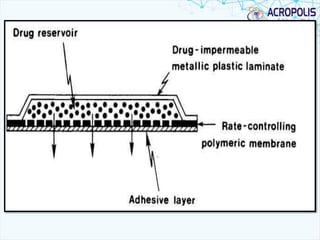
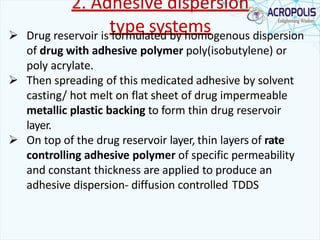
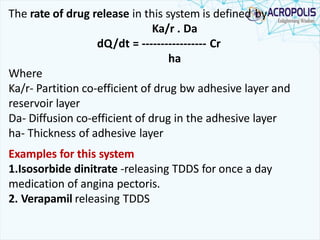
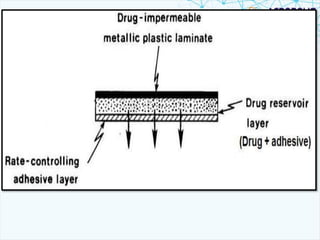
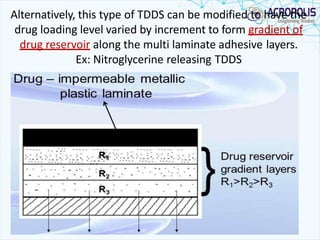
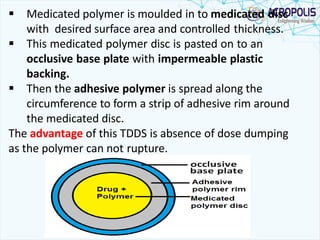
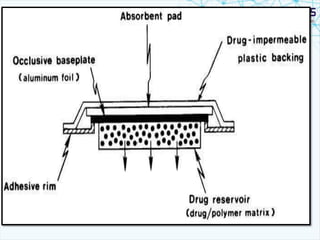
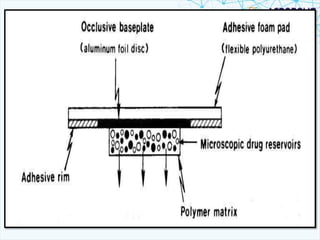
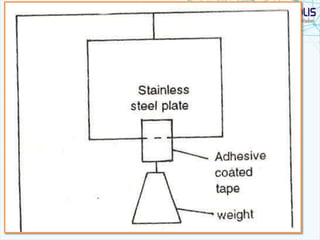
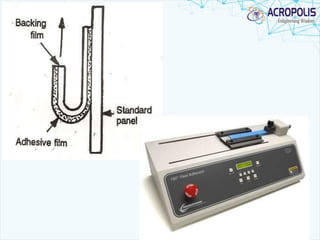
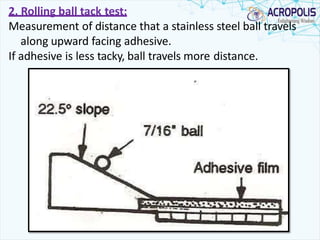
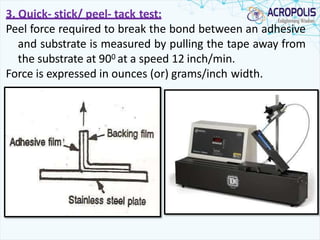
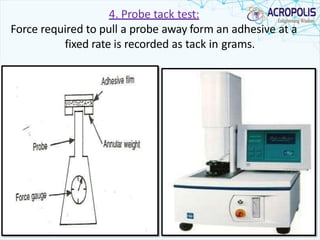
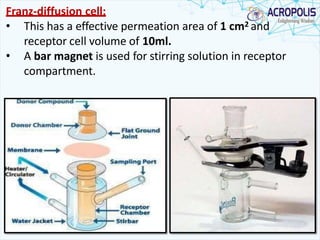
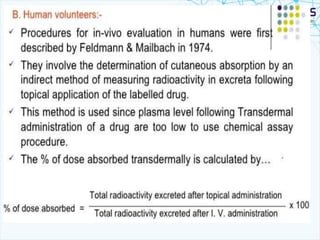
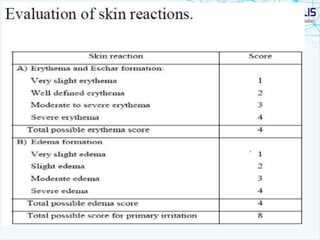
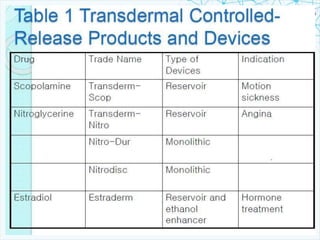
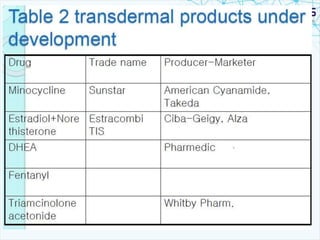
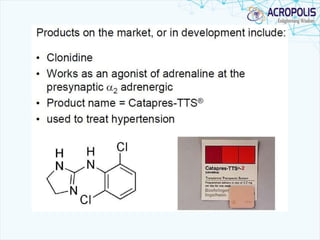
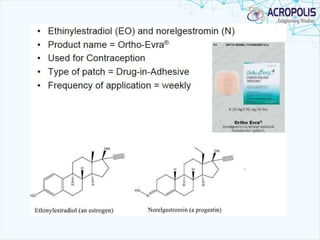
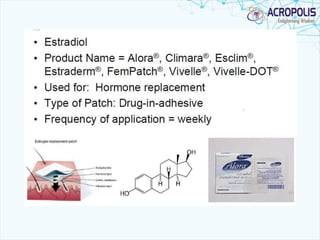
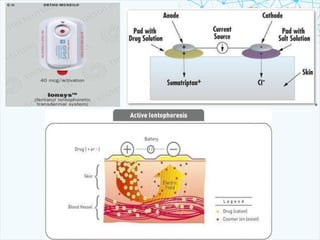
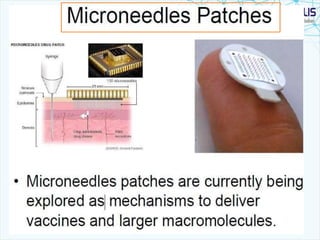
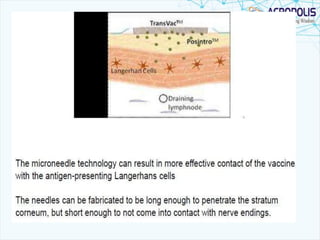
This document discusses transdermal drug delivery systems. It provides information on:
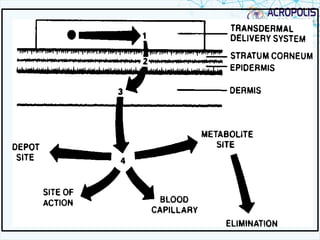
1. Transdermal drug delivery involves administering therapeutic agents through intact skin for systemic effects. Only a small number of drug products are currently available via this route.
2. The first transdermal patch was approved in 1981 to prevent nausea and vomiting from motion sickness. By 2003, the FDA had approved over 20 transdermal patch products containing 13 different drug molecules.
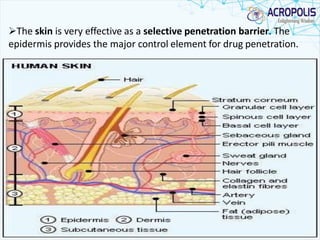
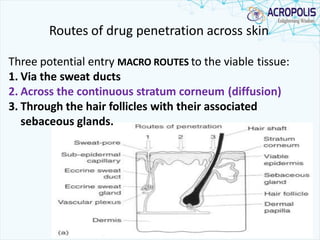
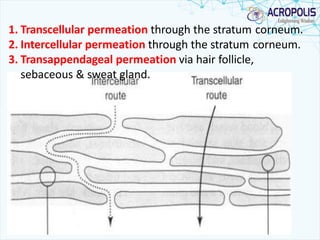
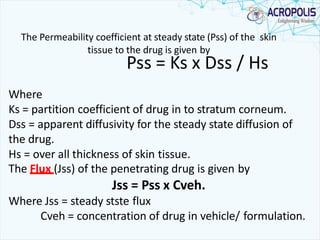
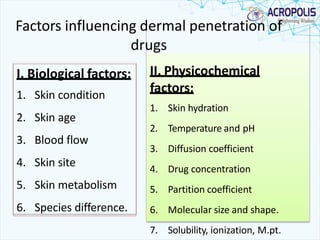
3. Successful transdermal delivery depends on a drug's physicochemical properties like molecular size and polarity. The skin provides a selective penetration barrier primarily through the epidermis.