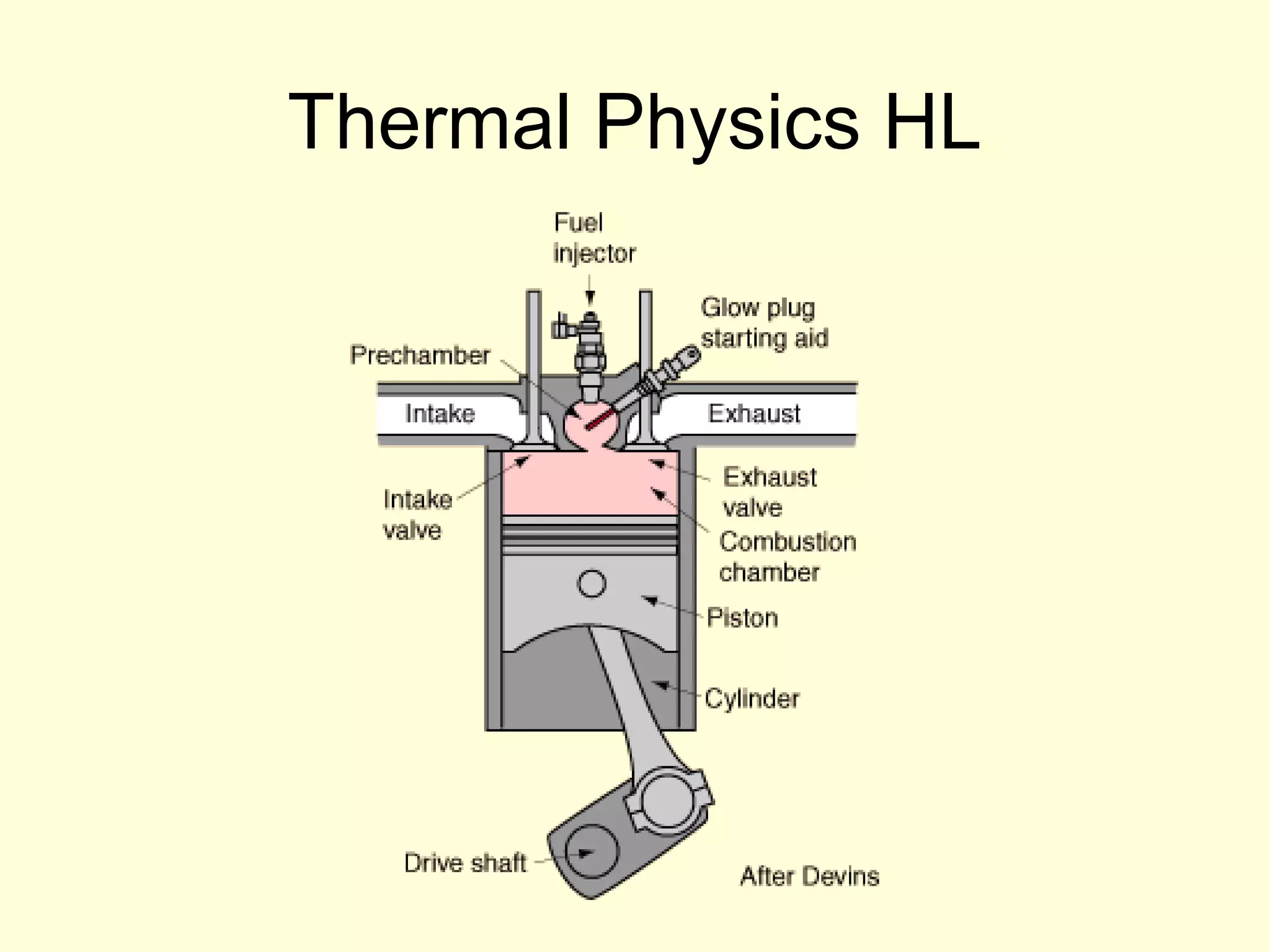
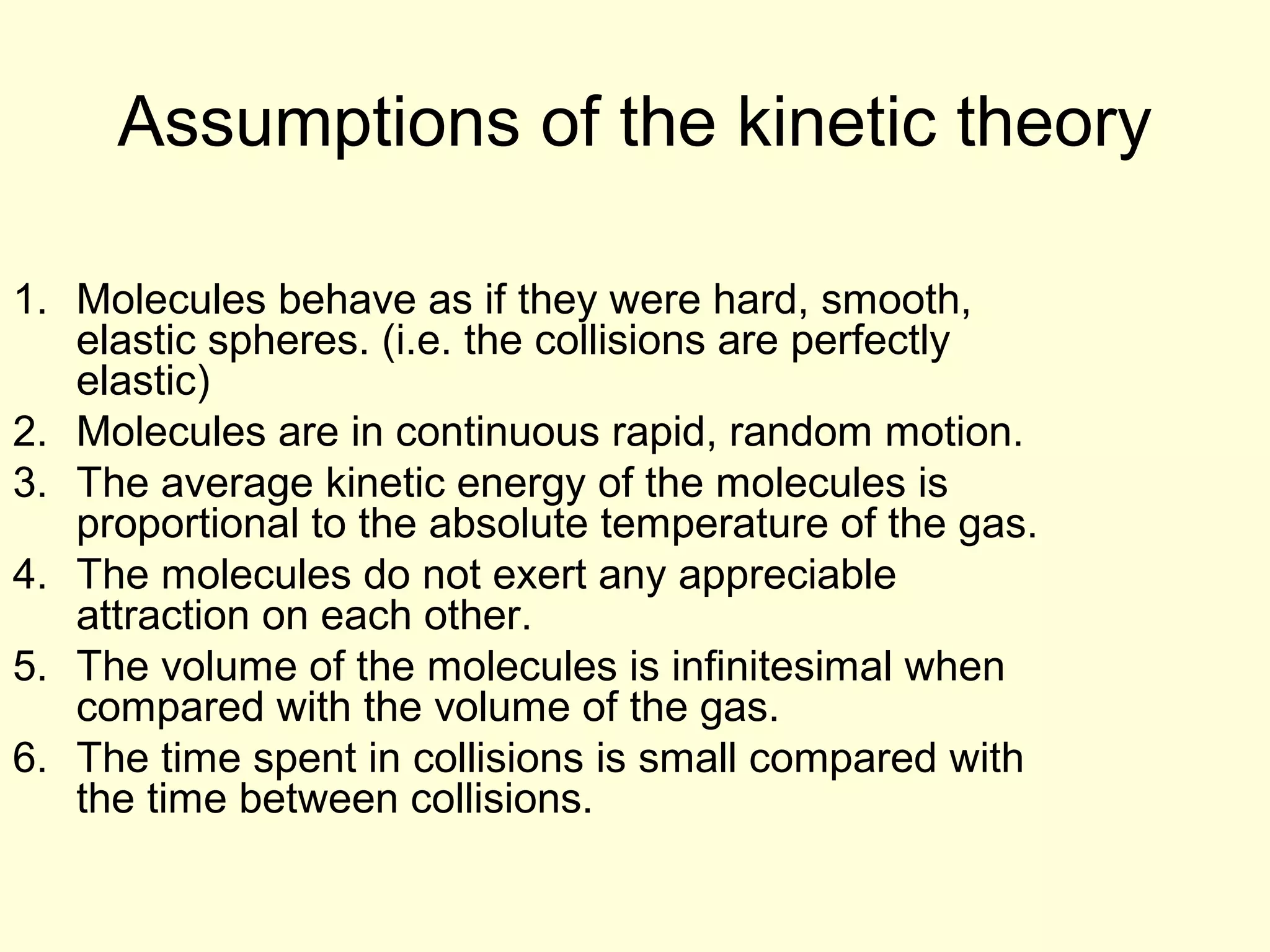
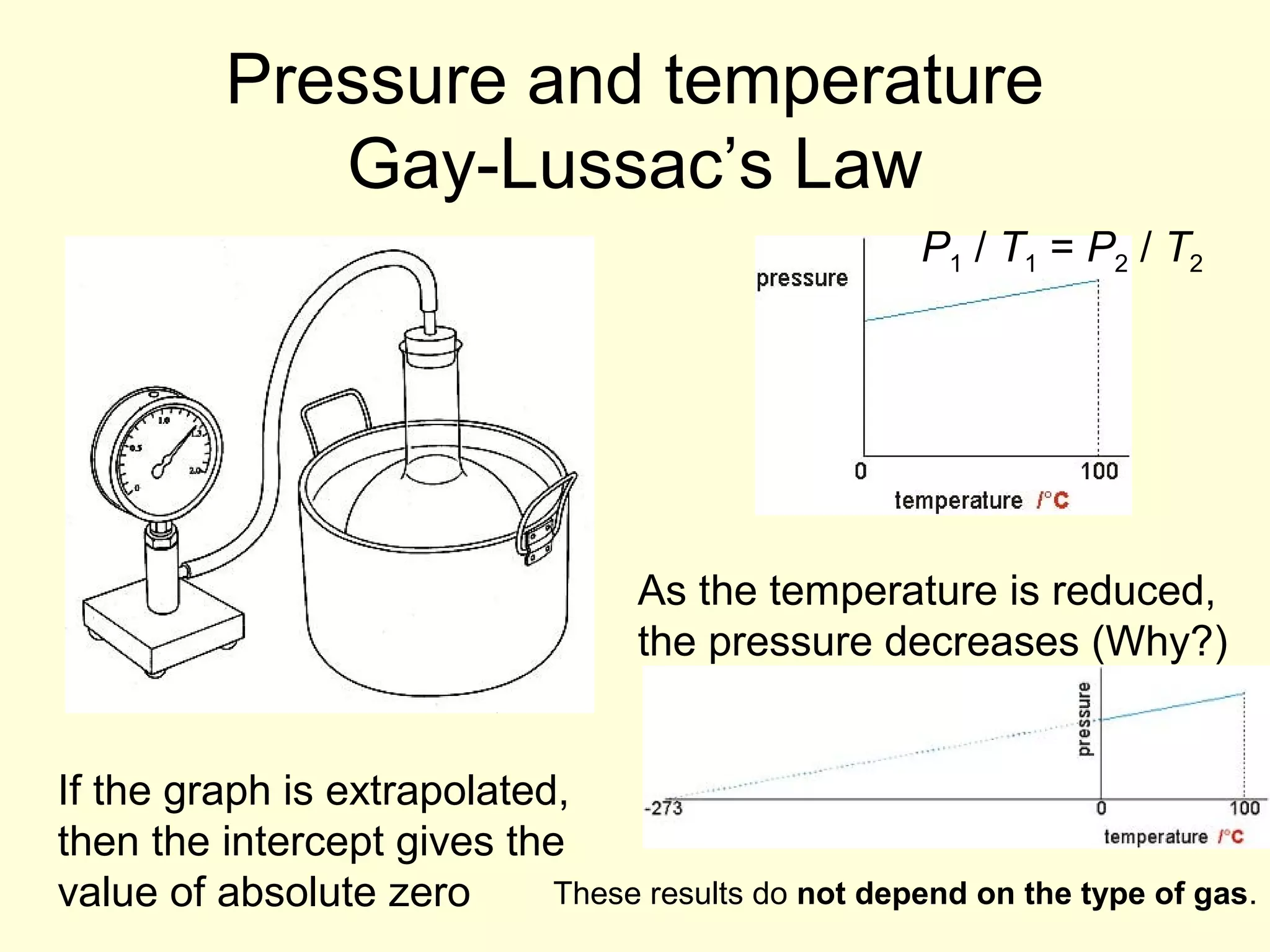
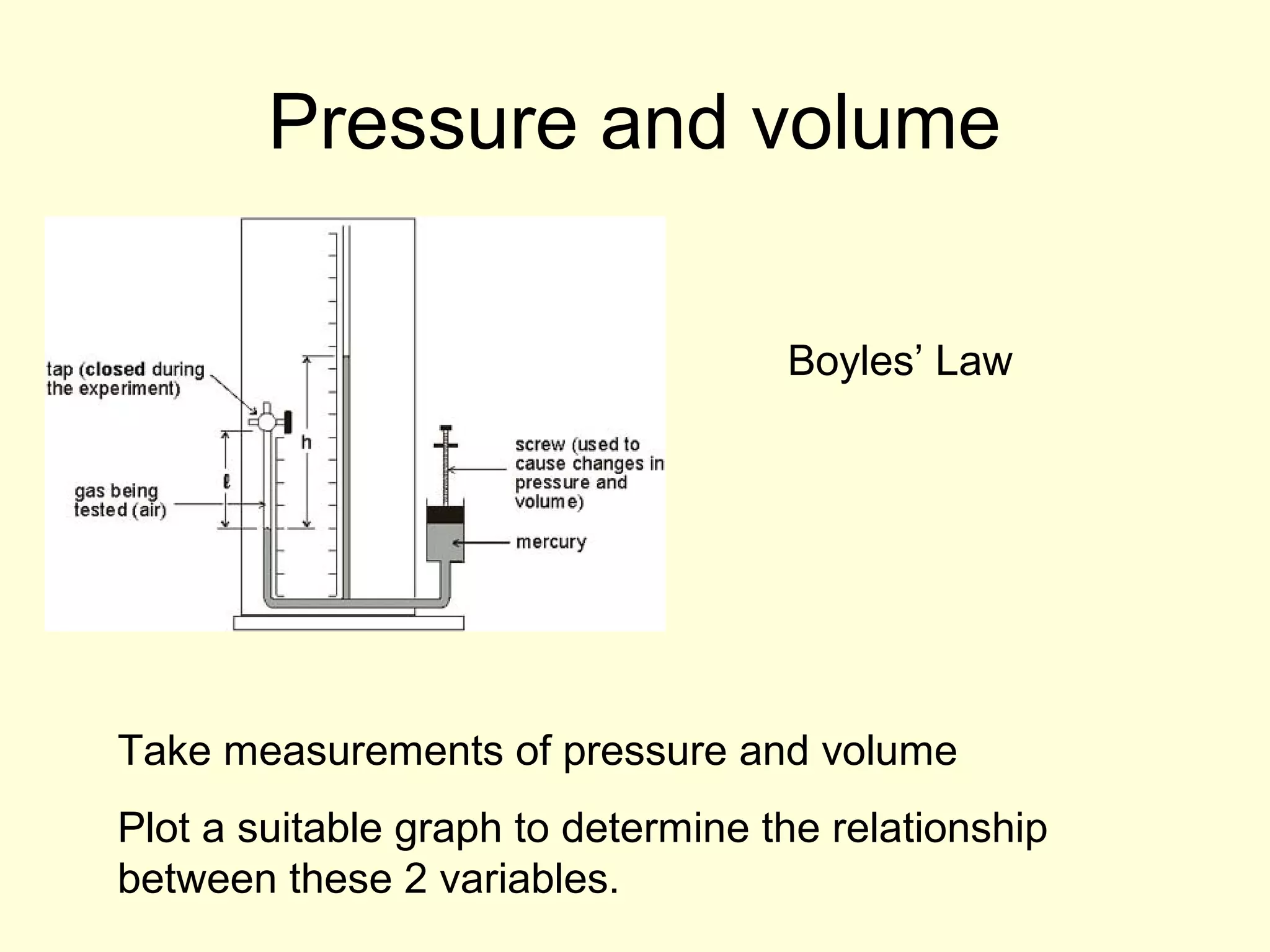
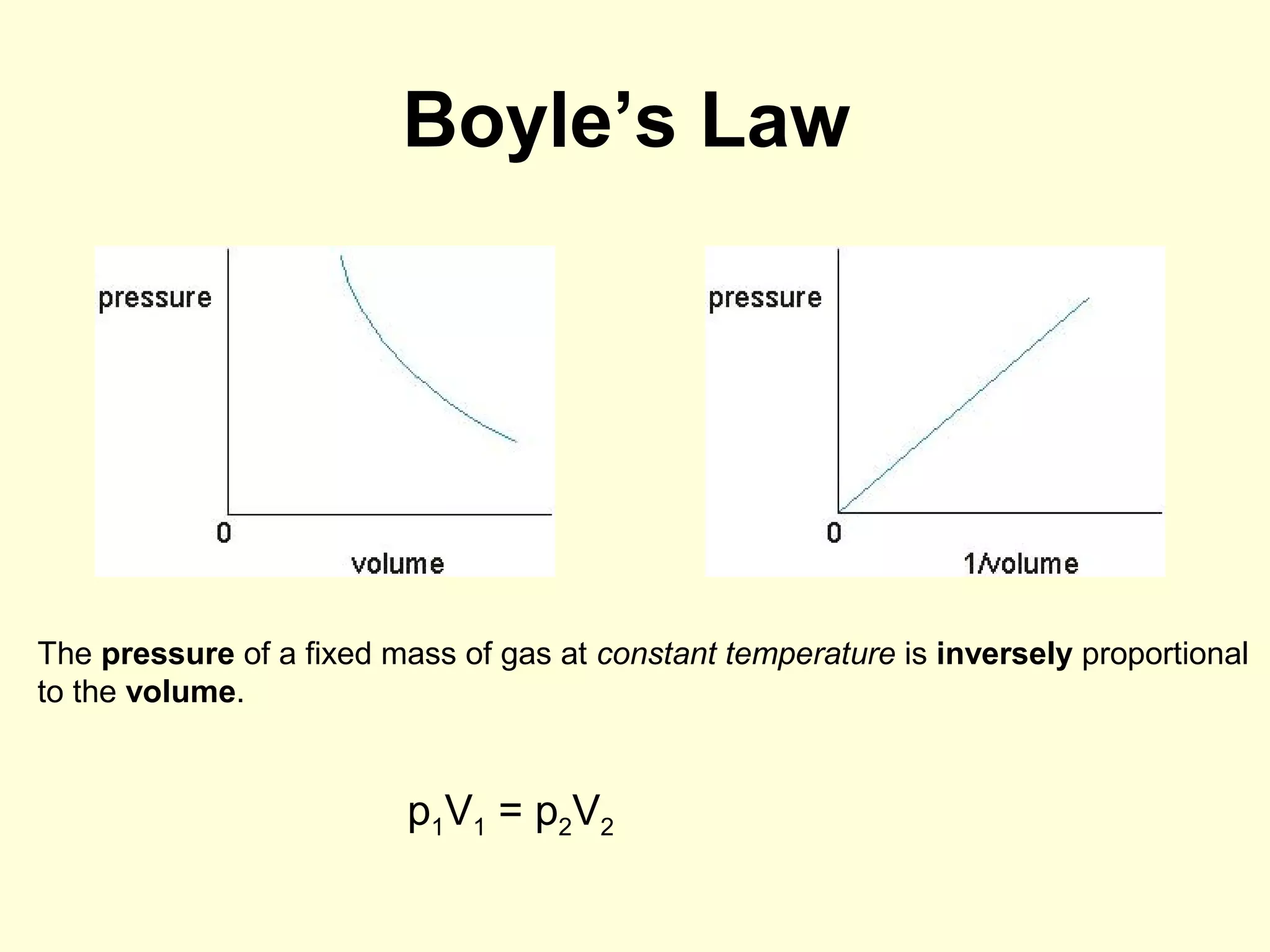
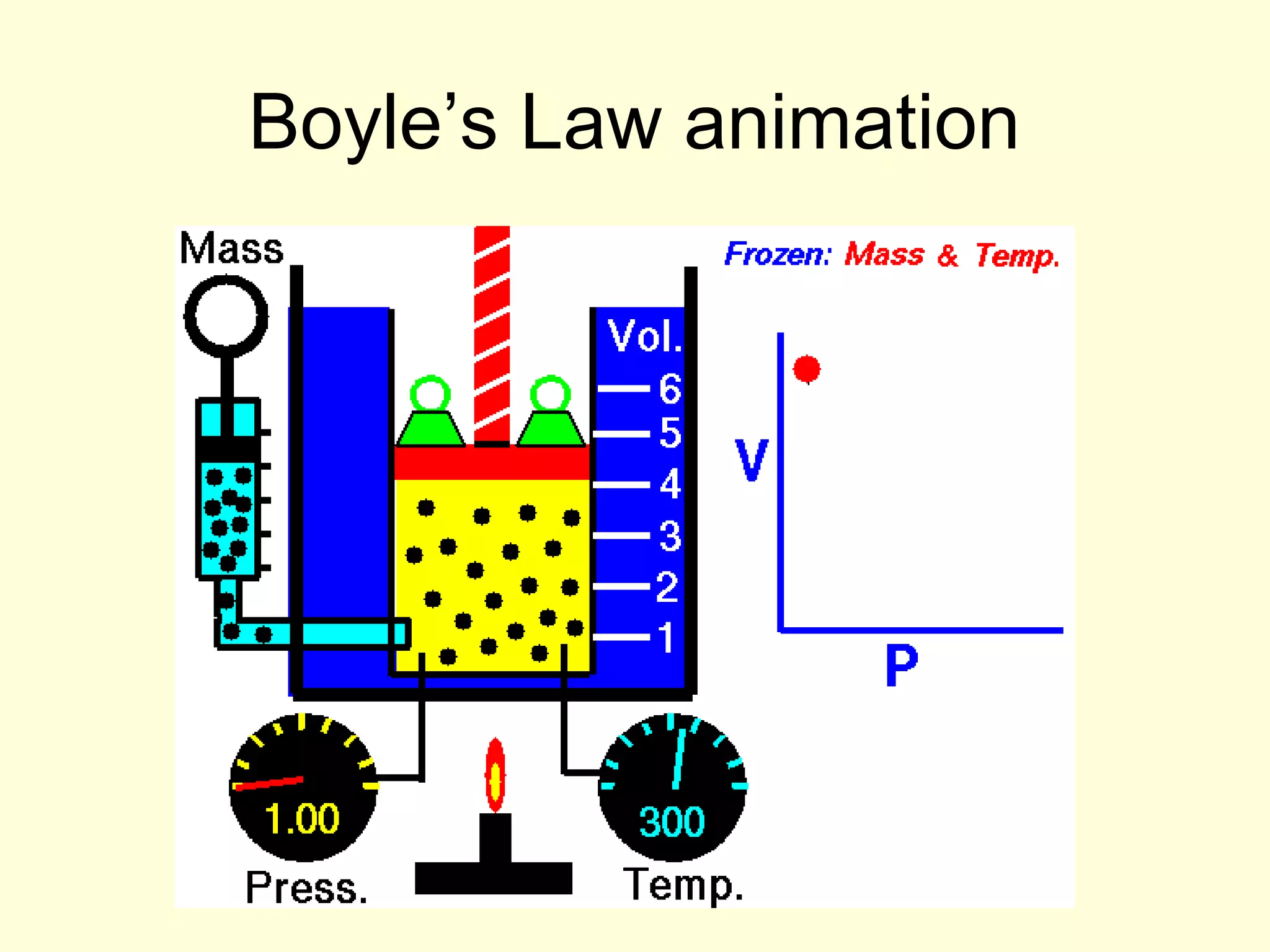
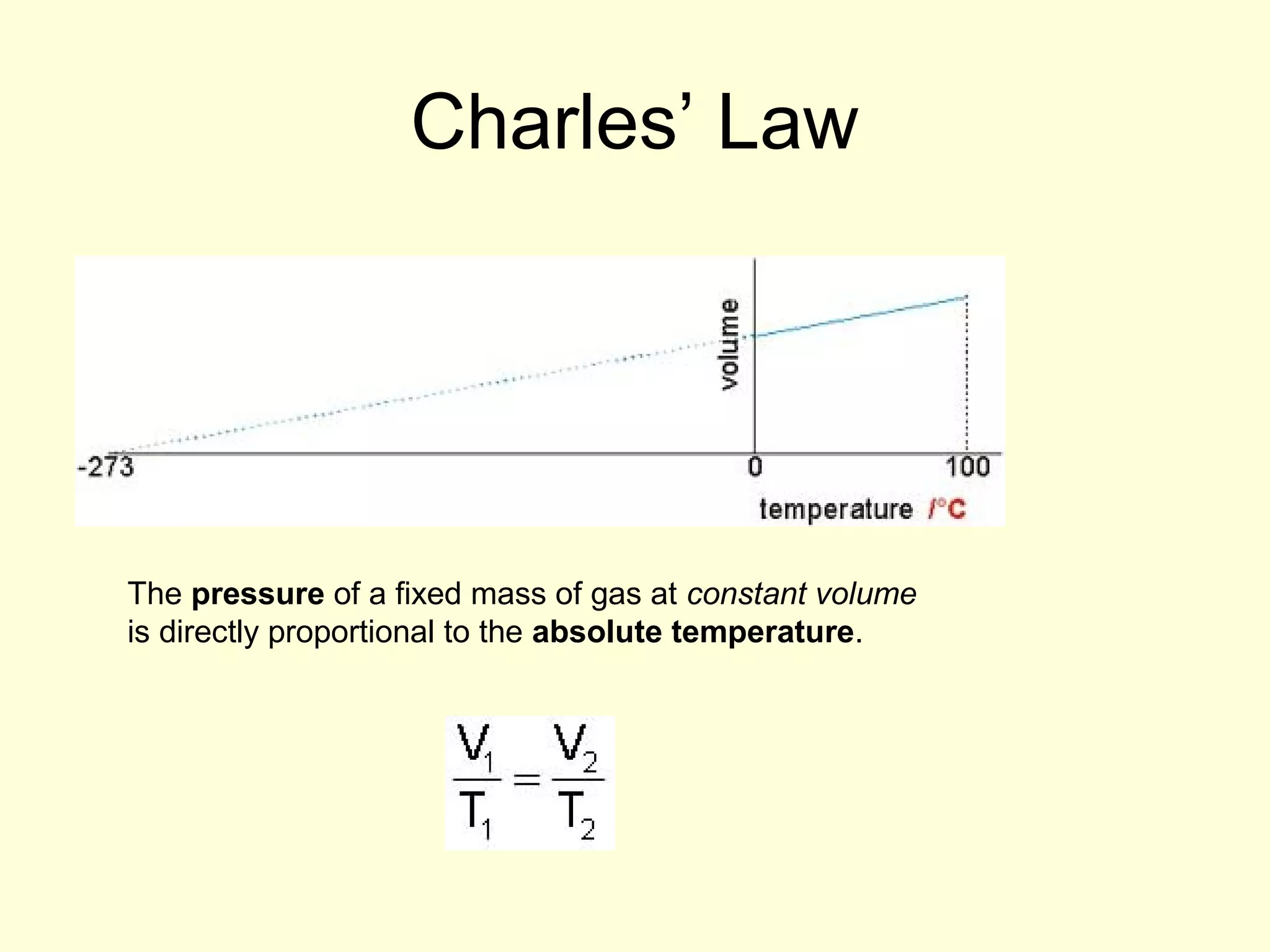
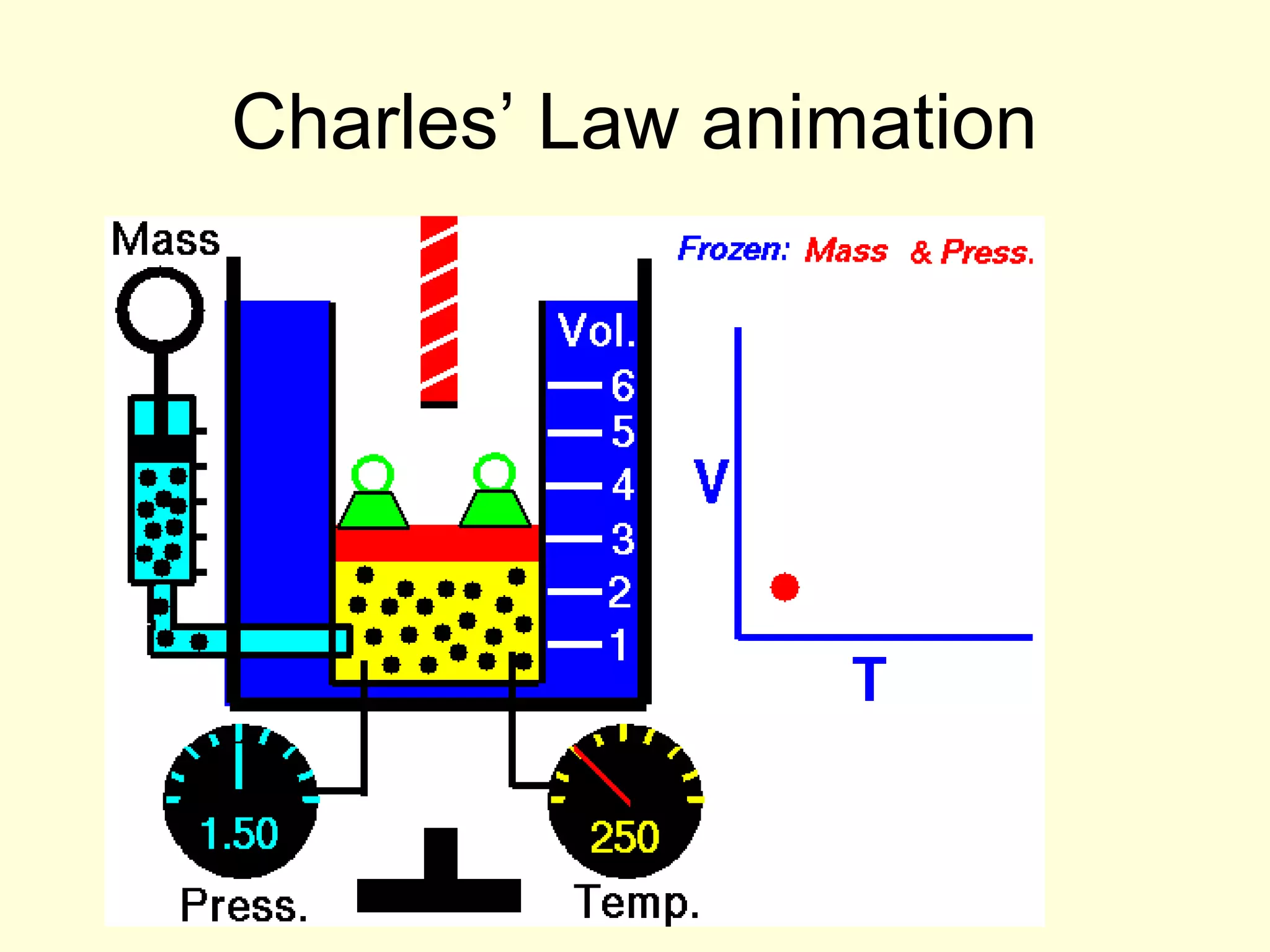
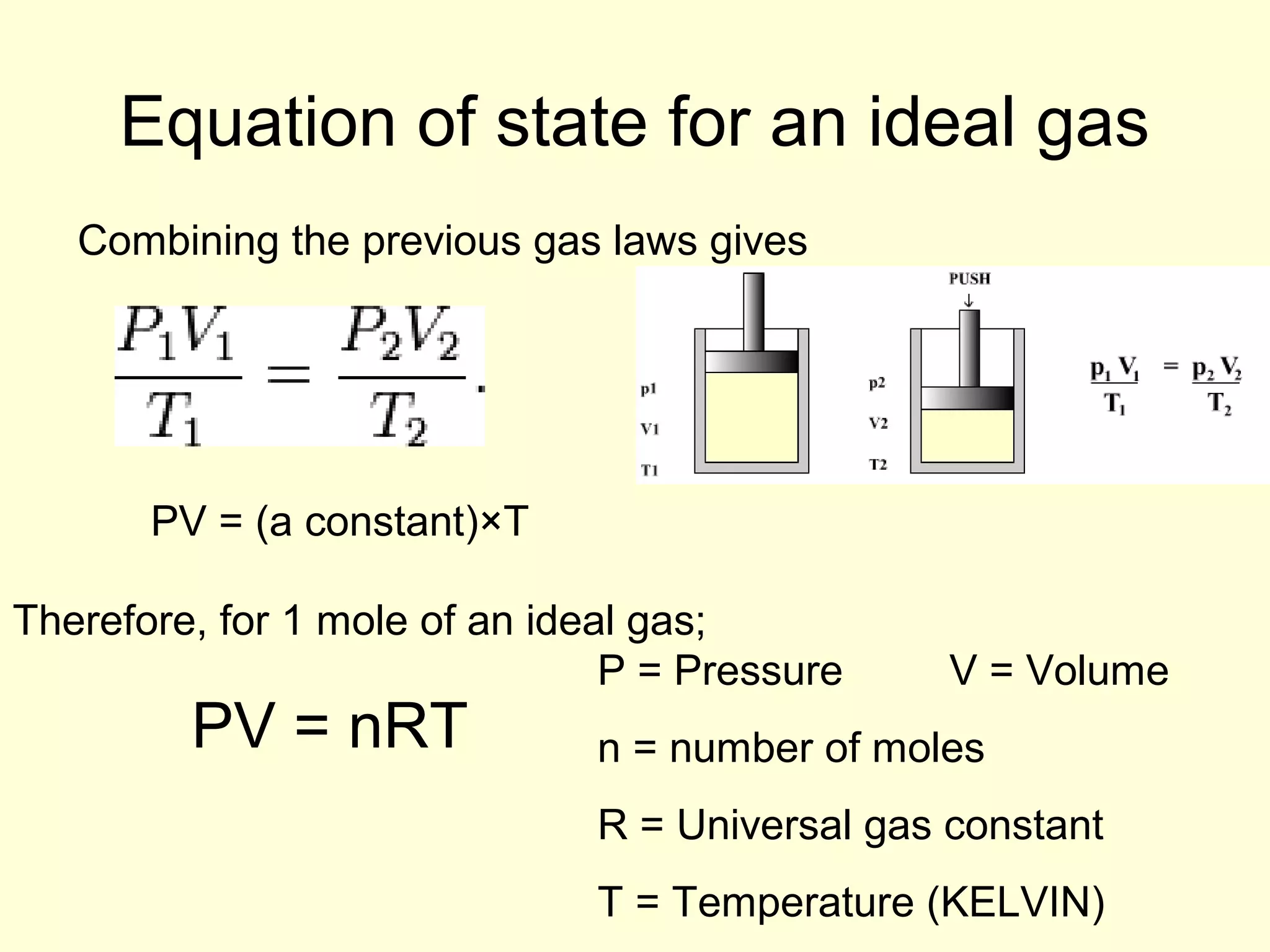
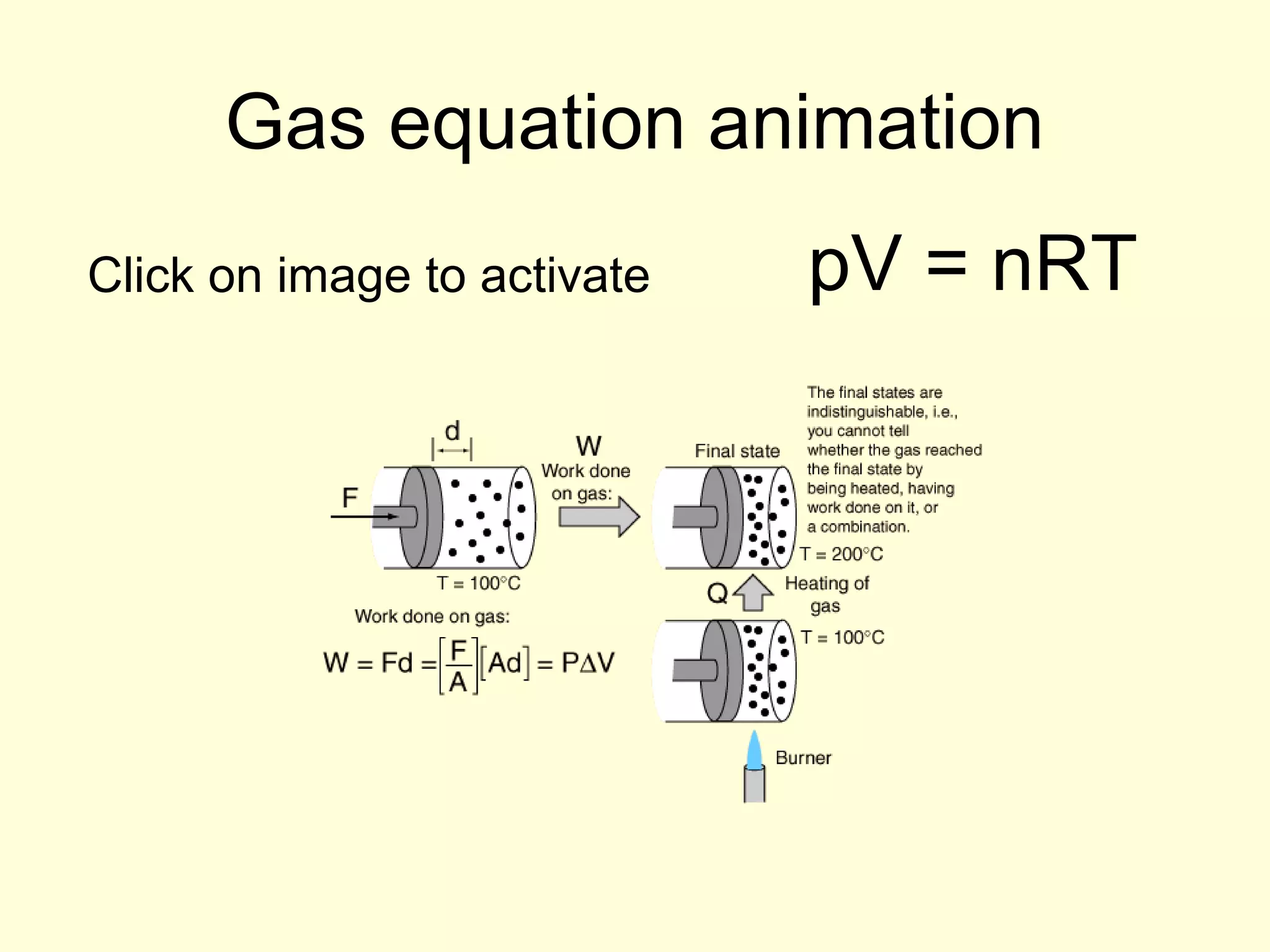
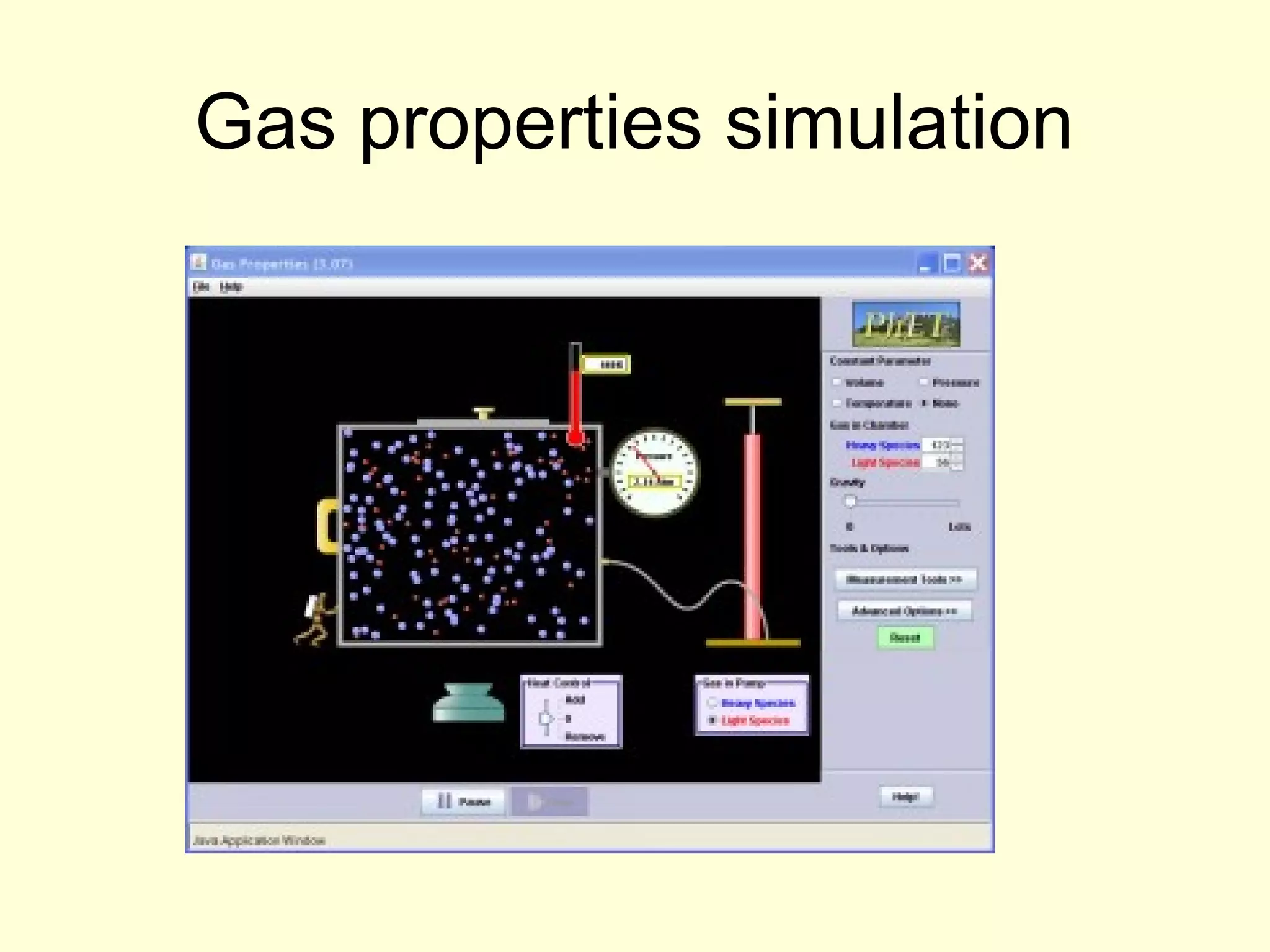
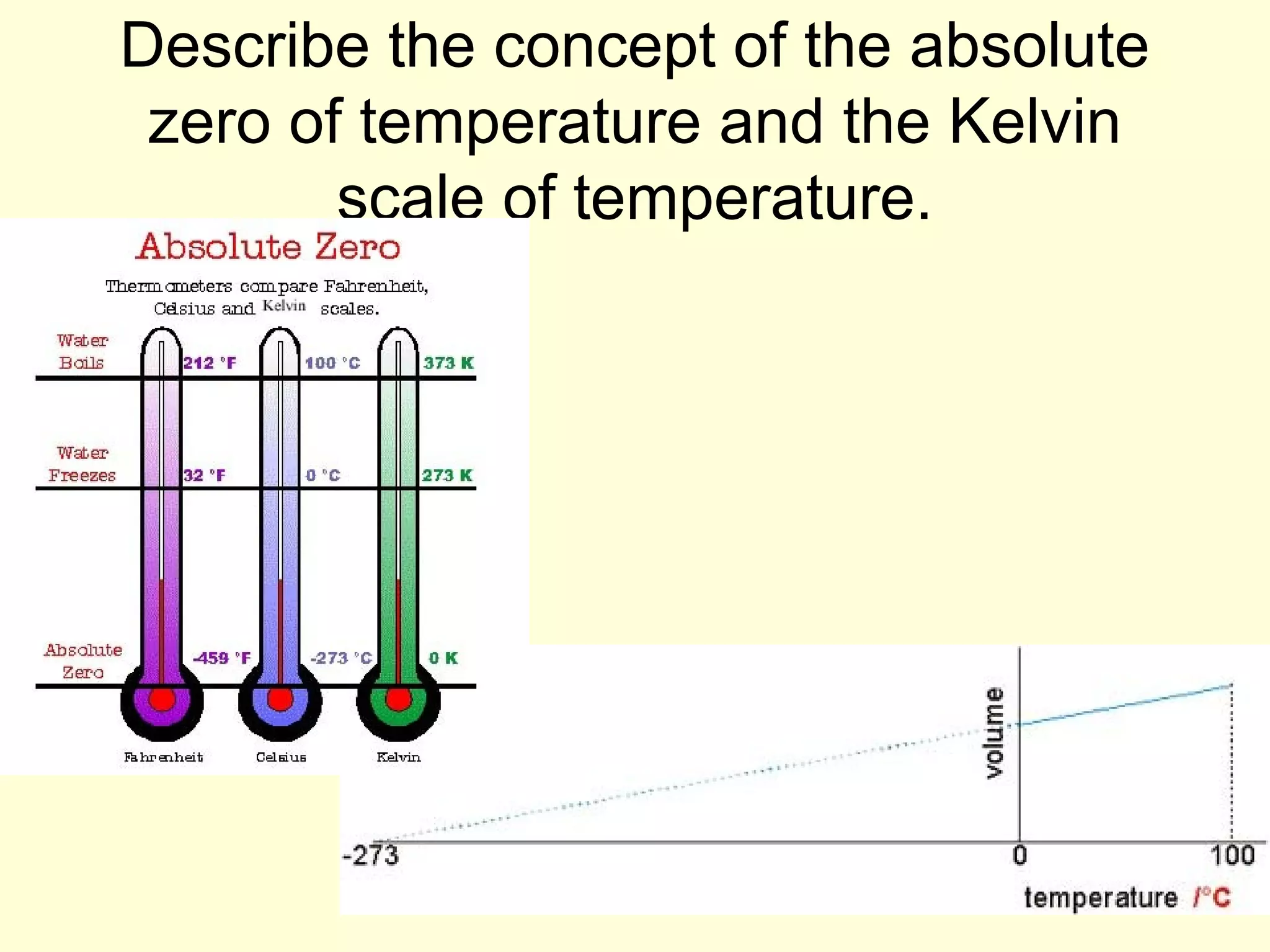
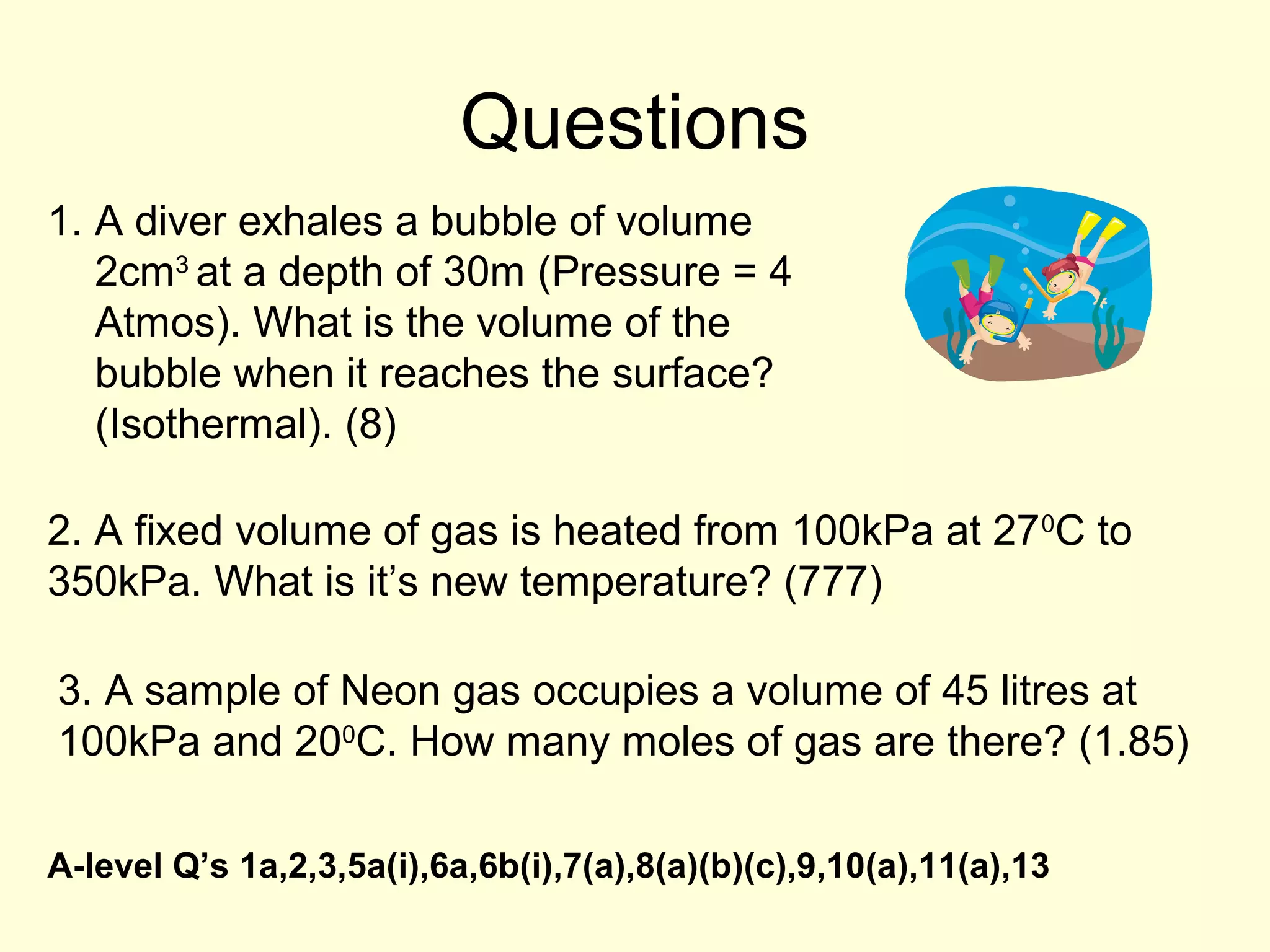
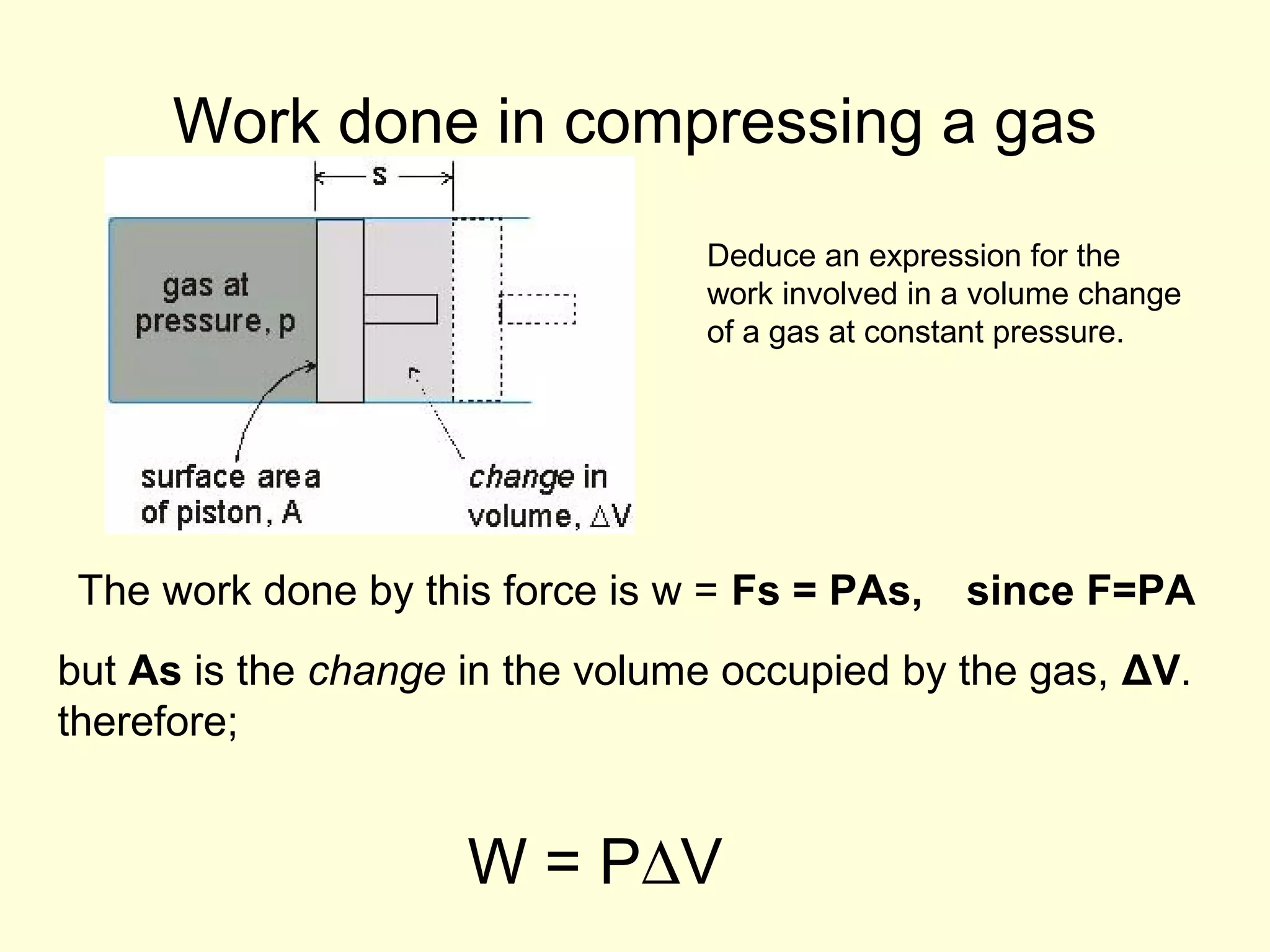
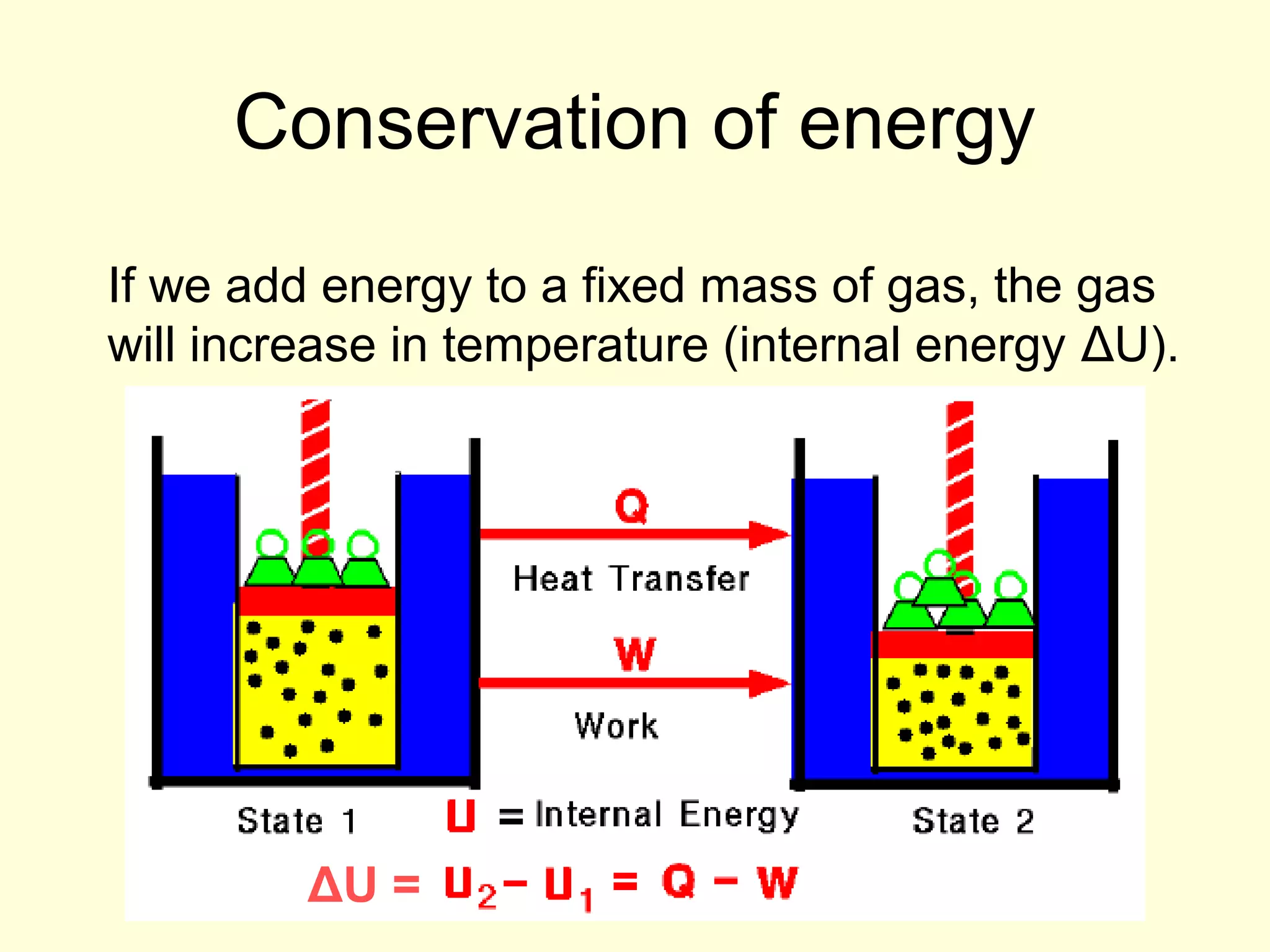
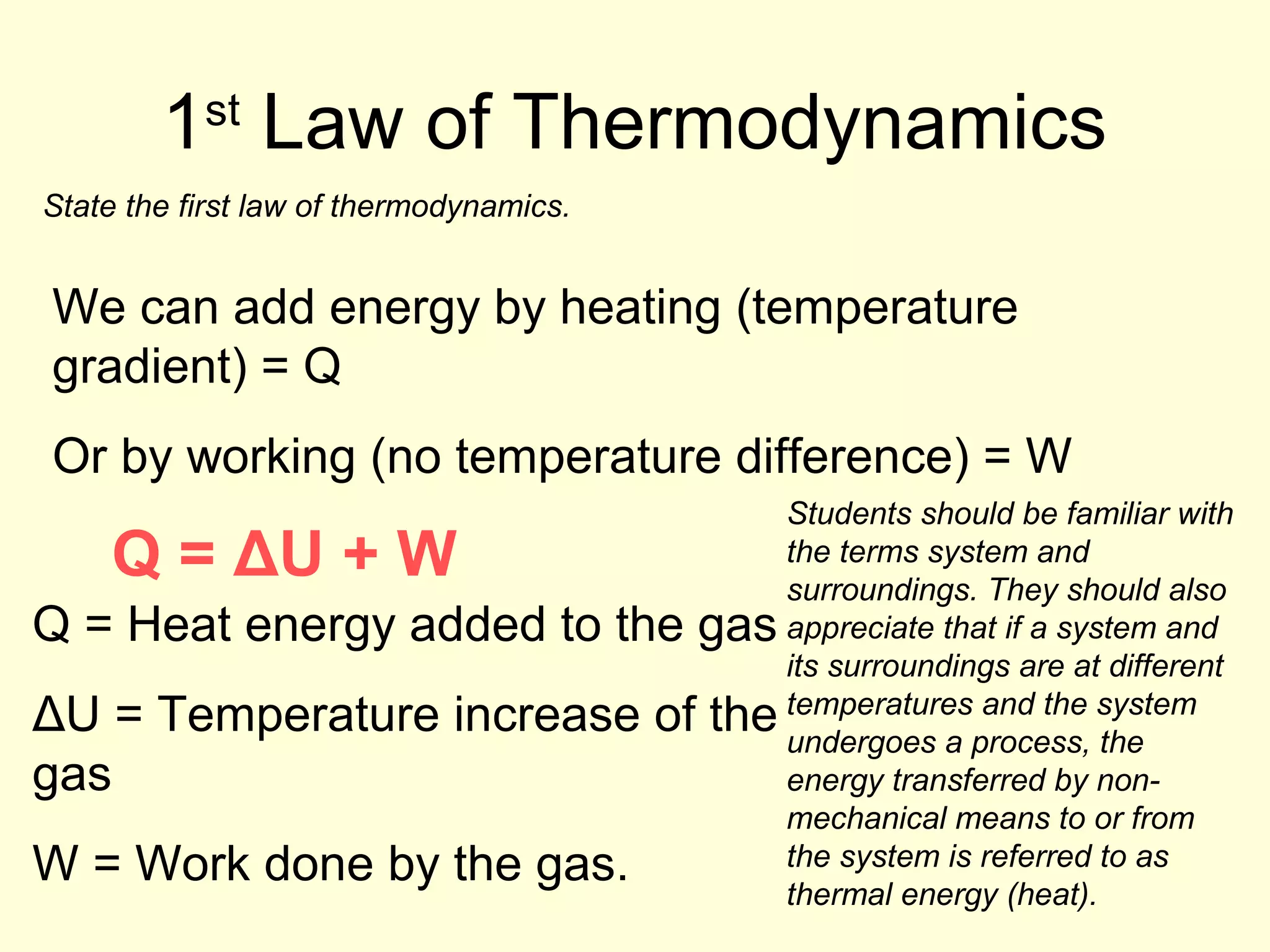
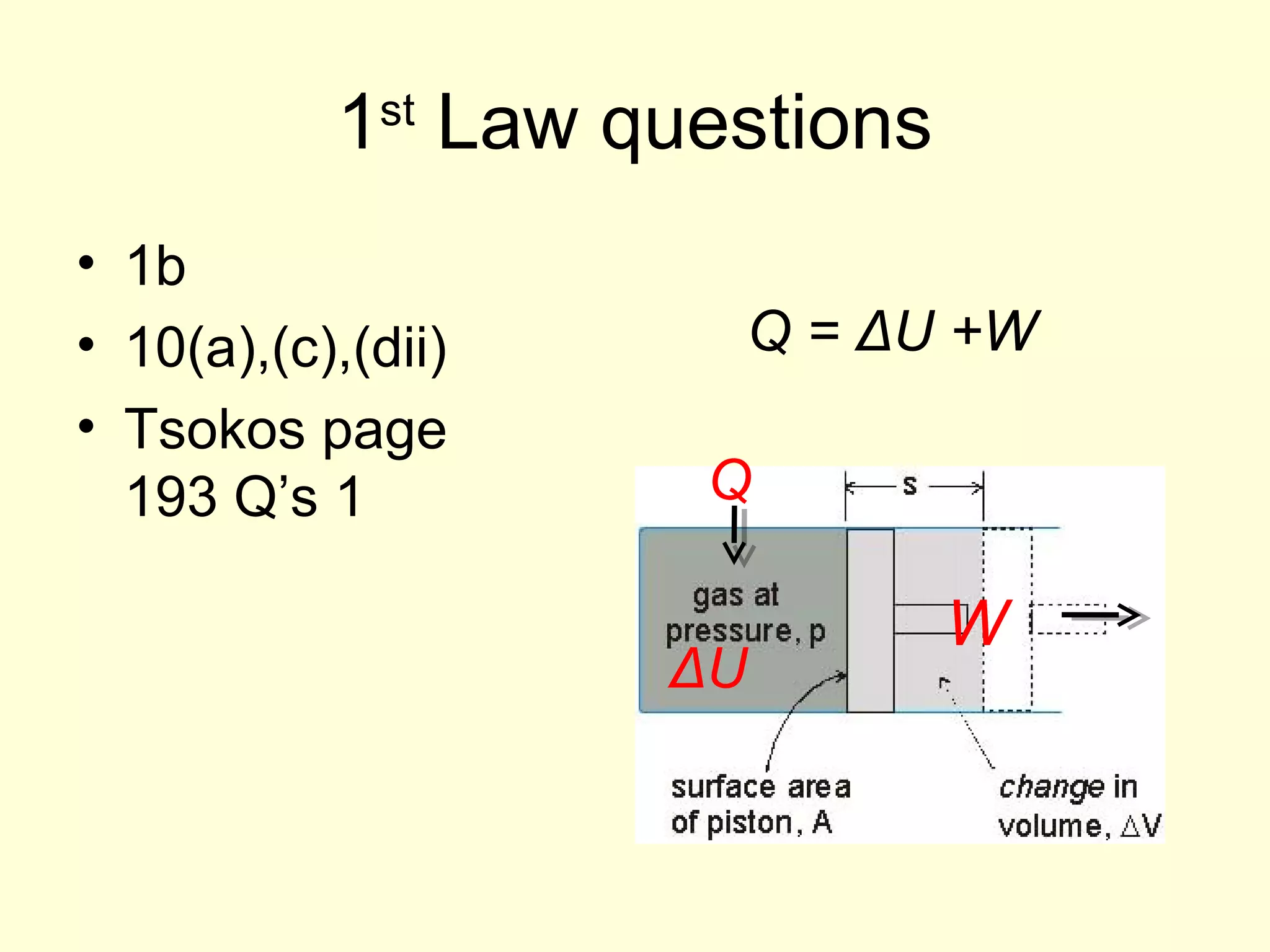
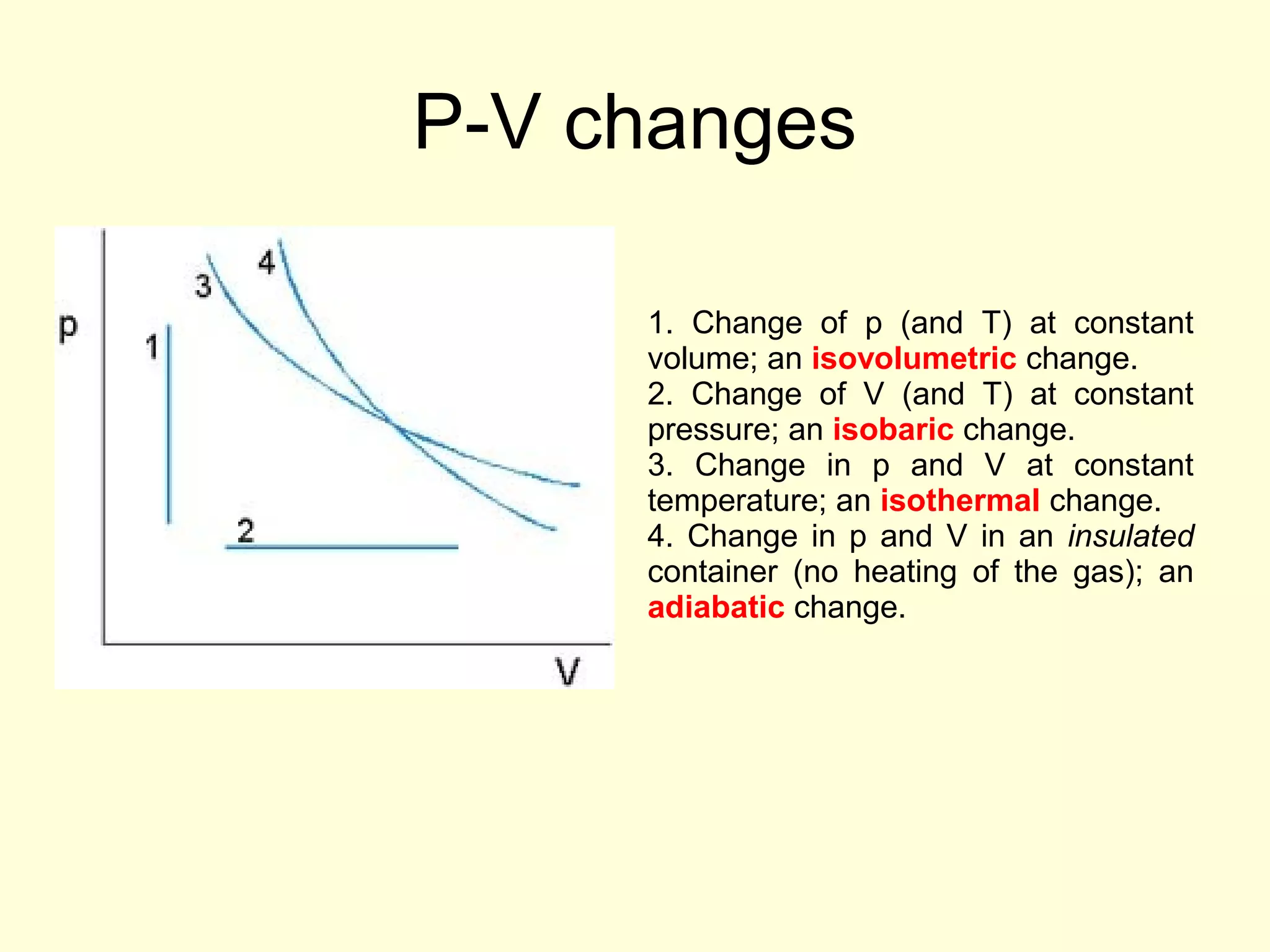
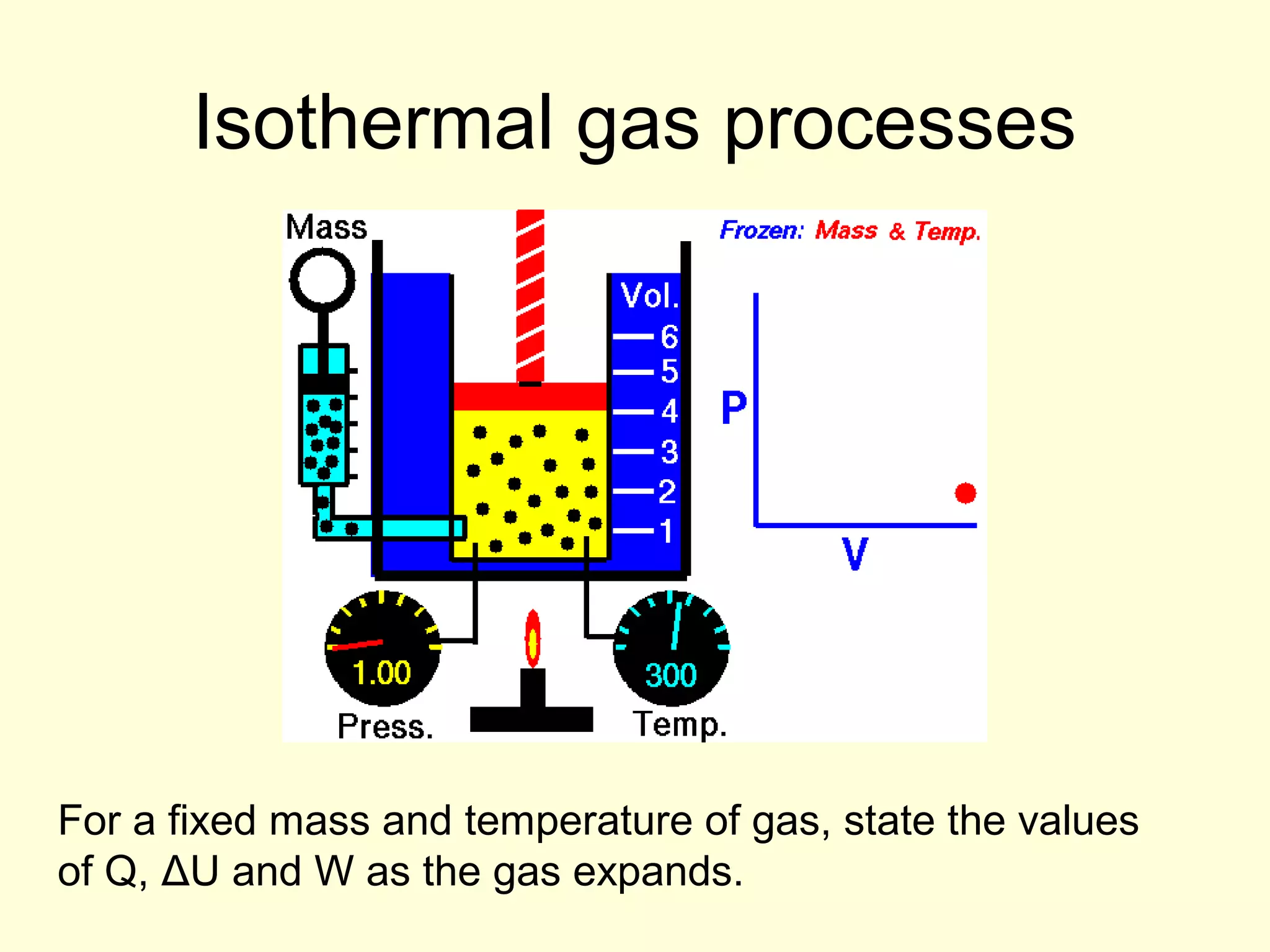
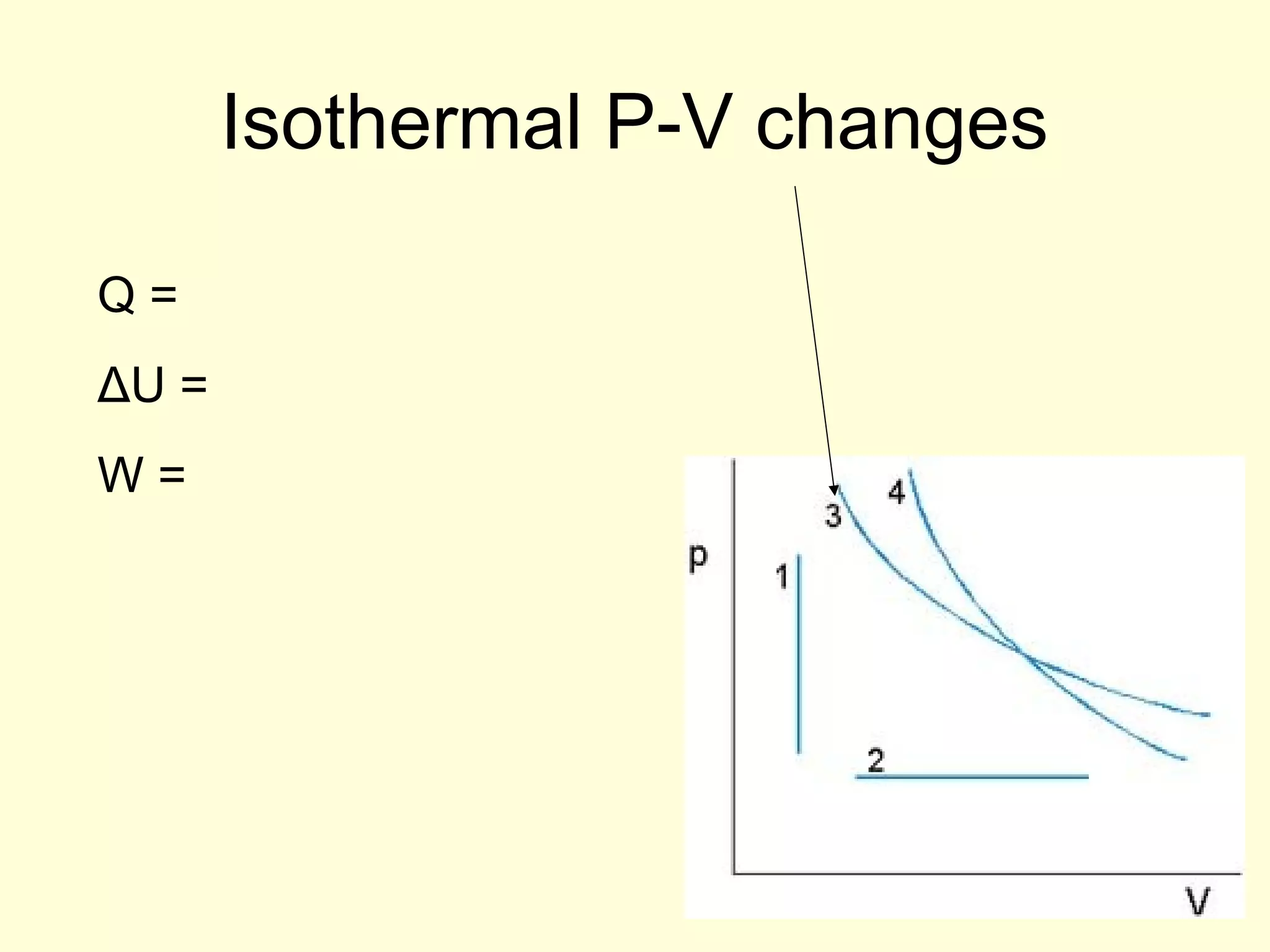
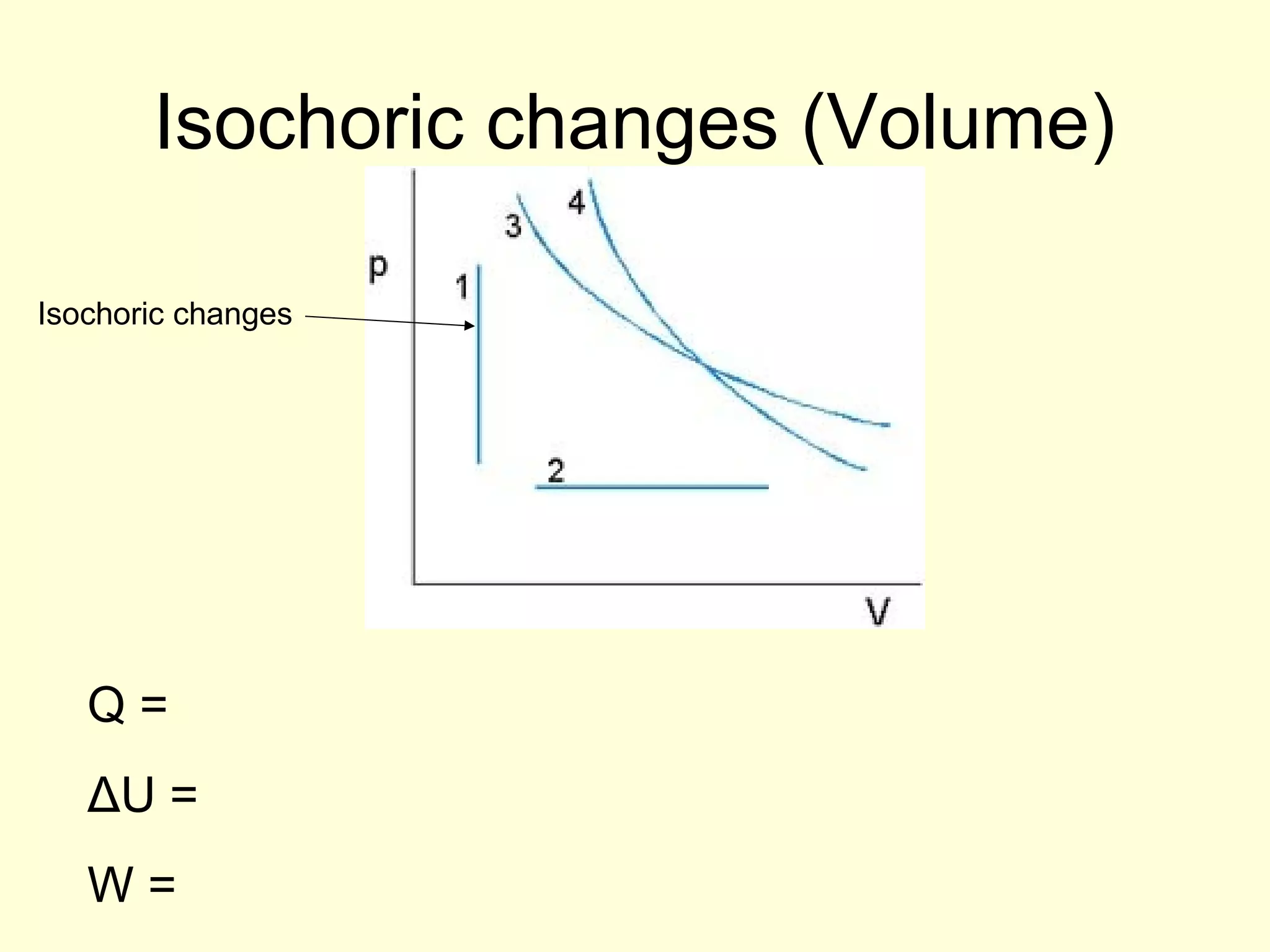
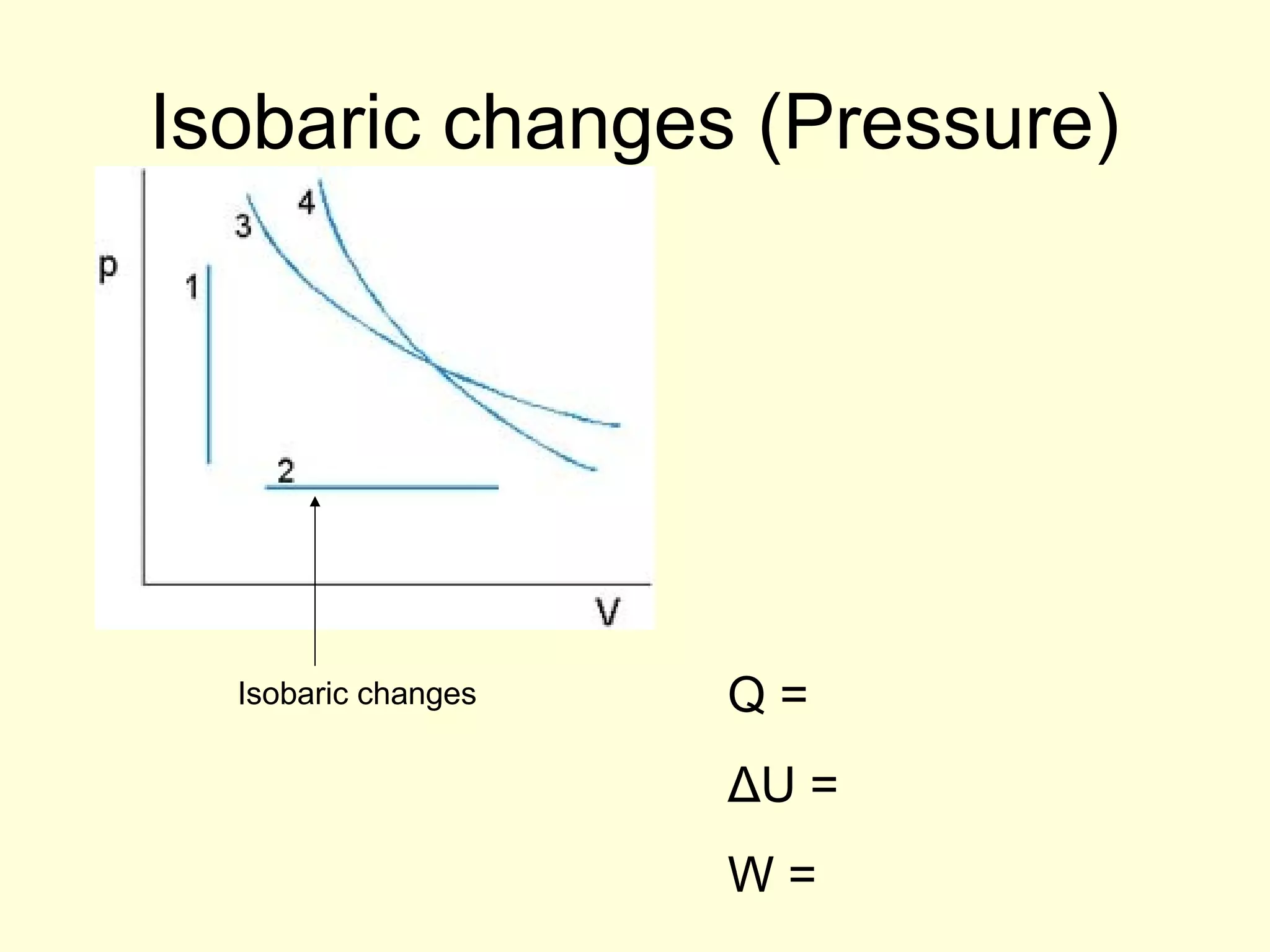
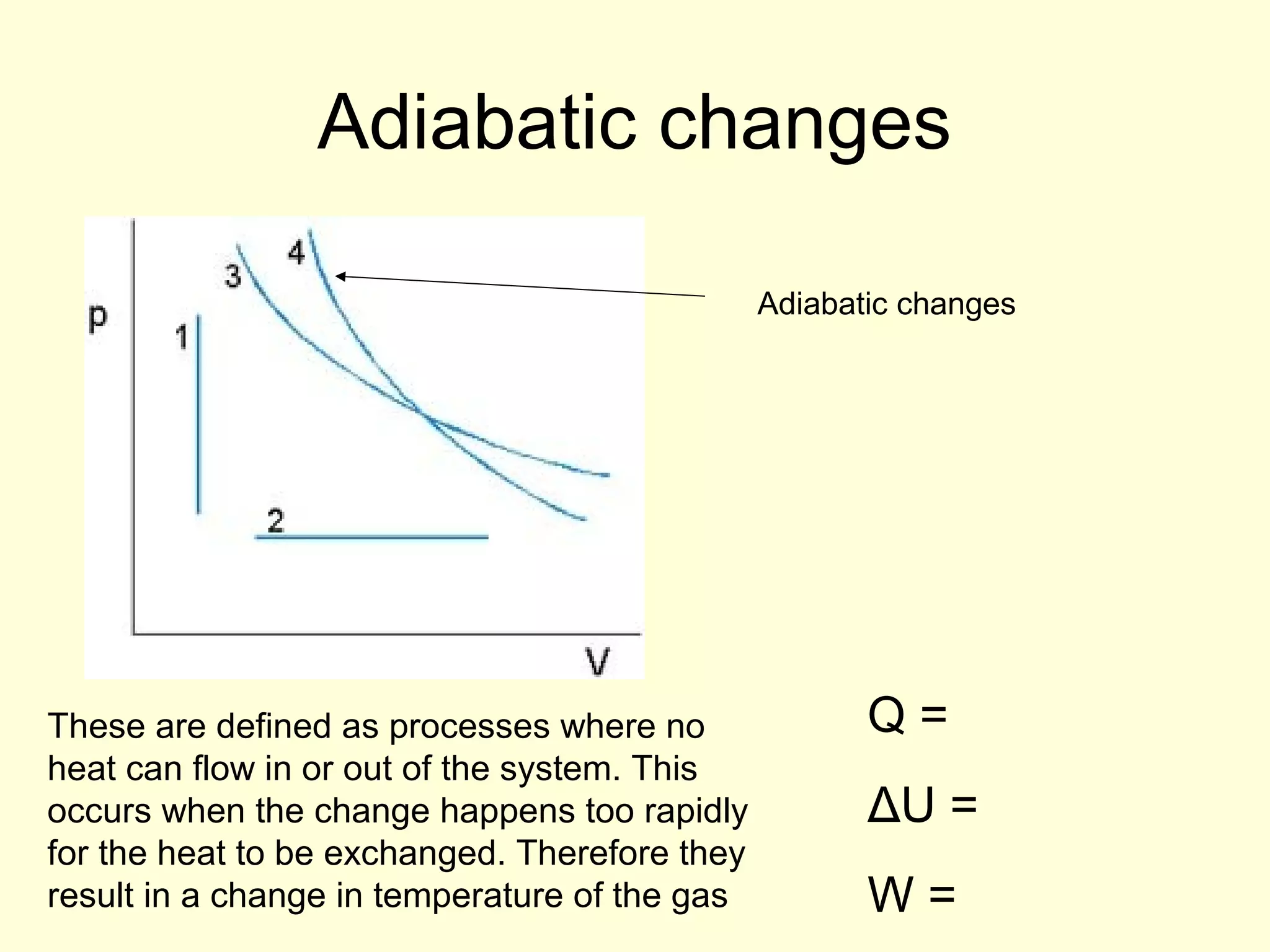
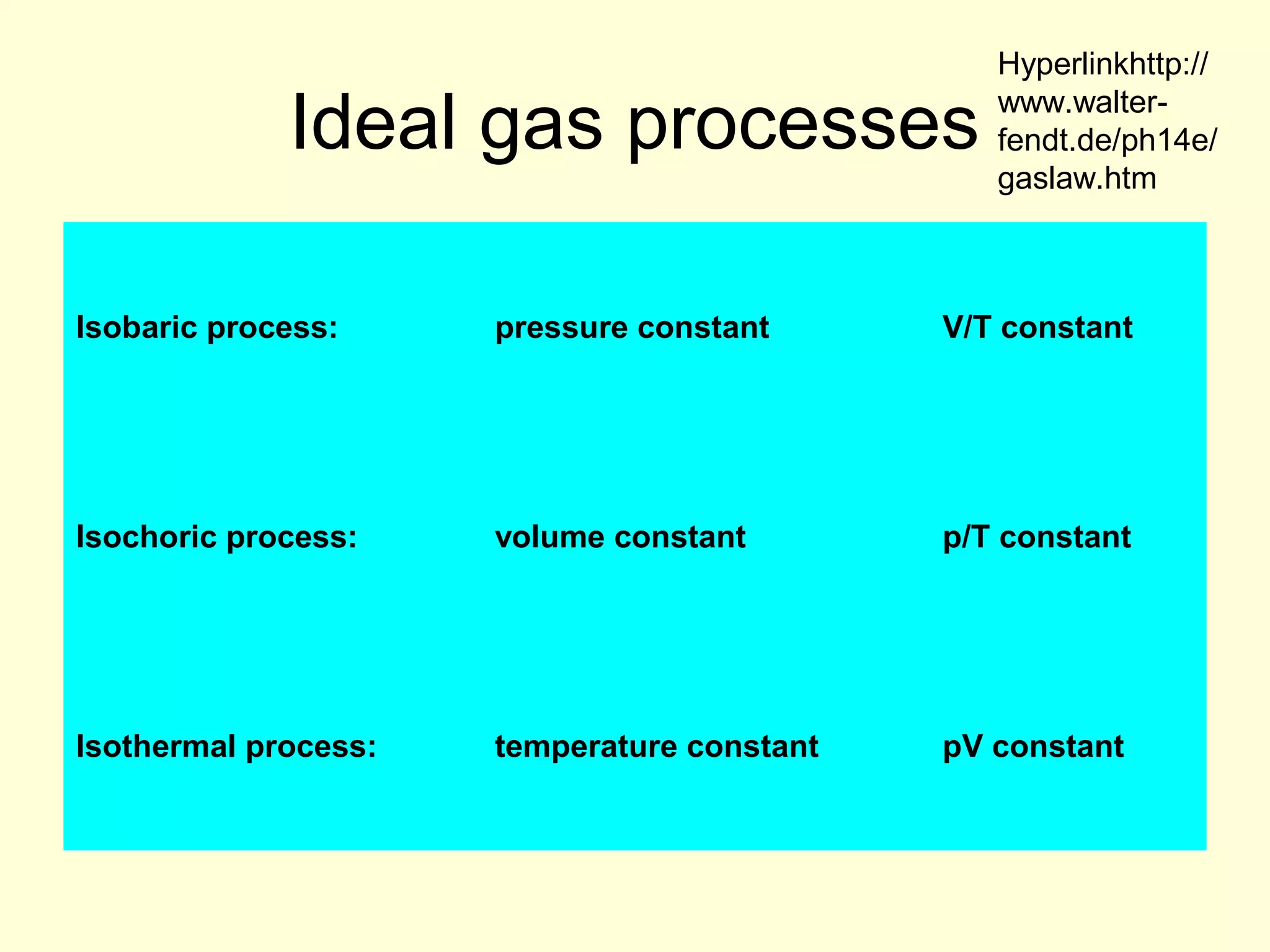
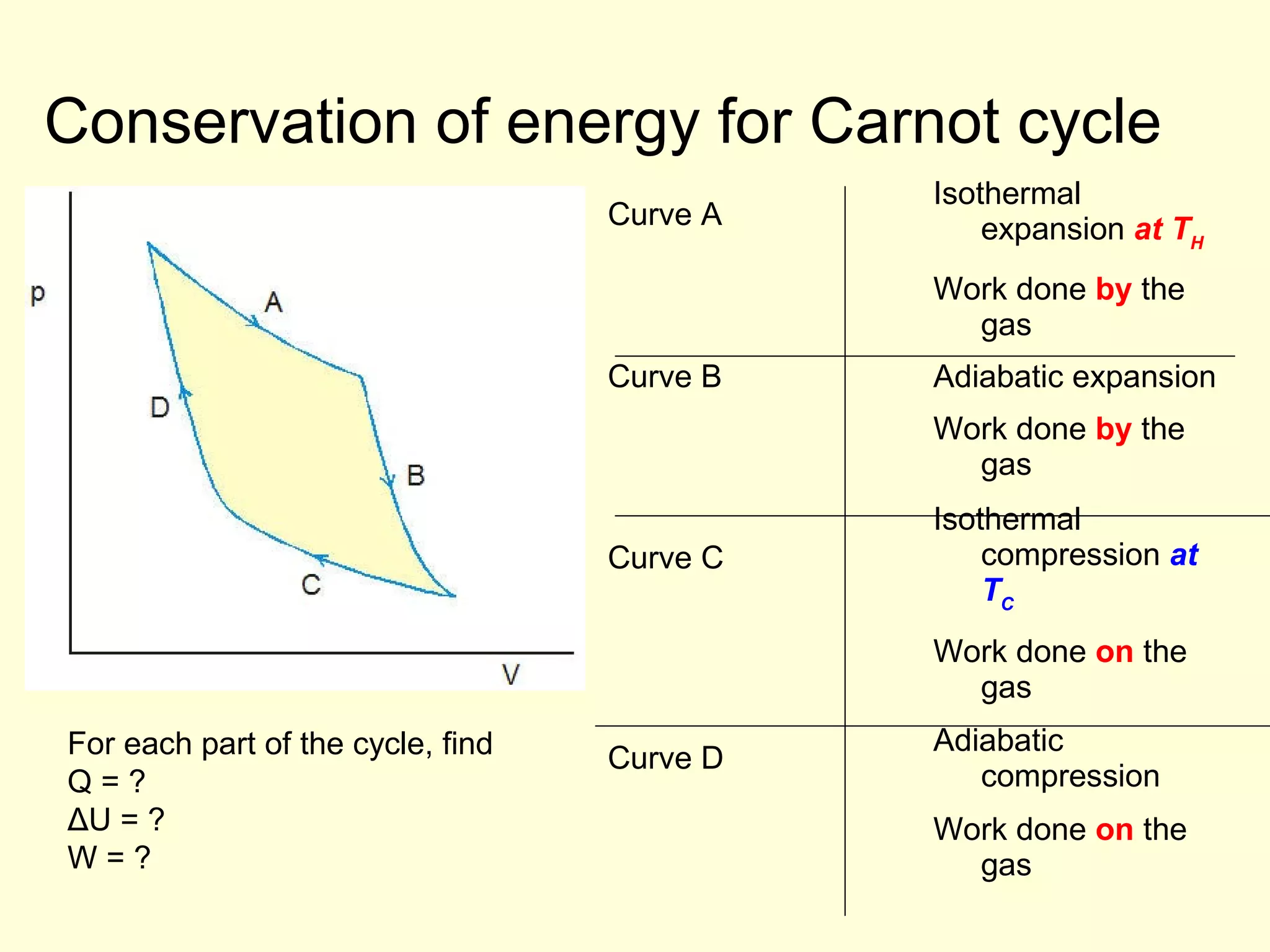
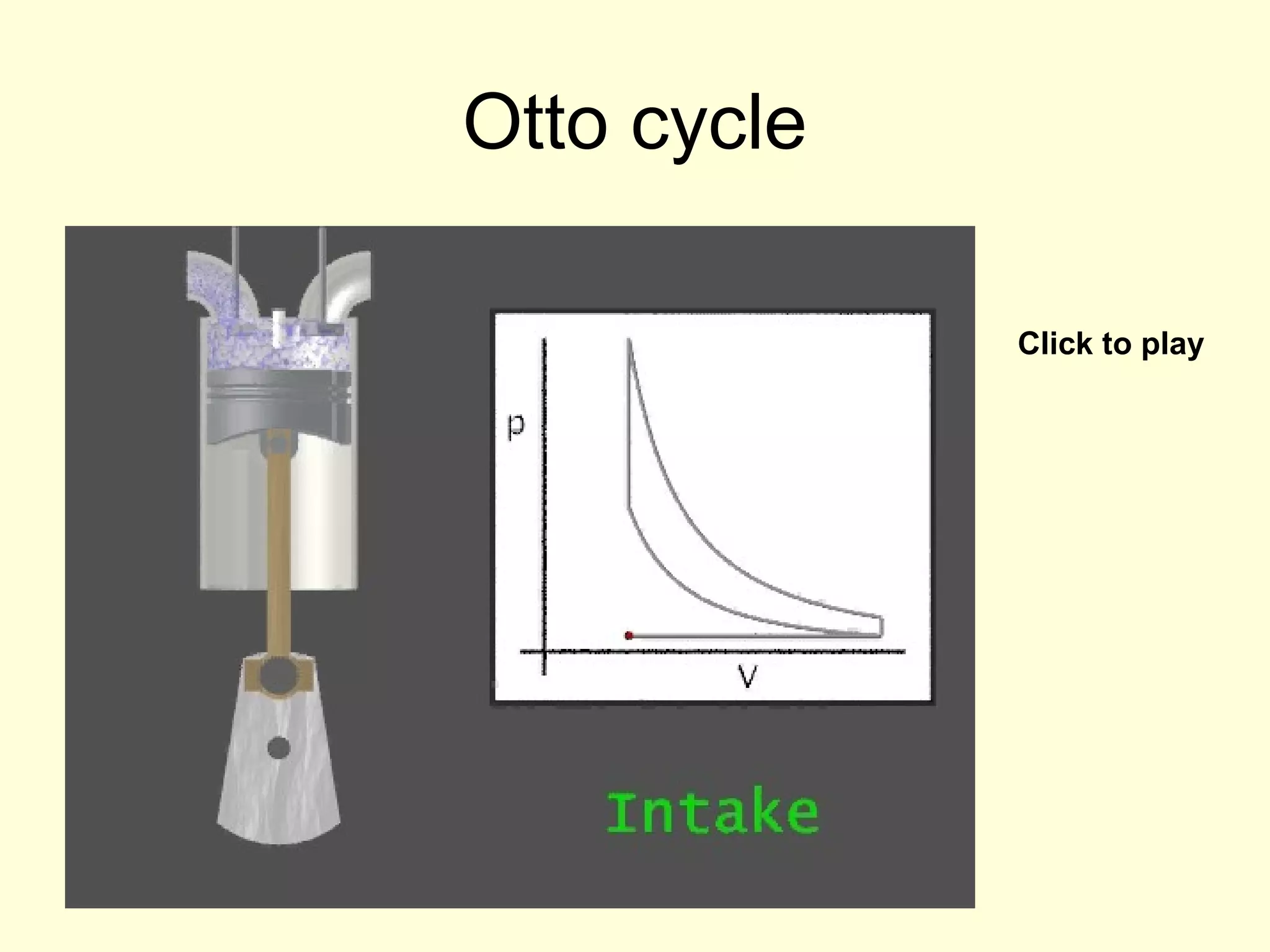
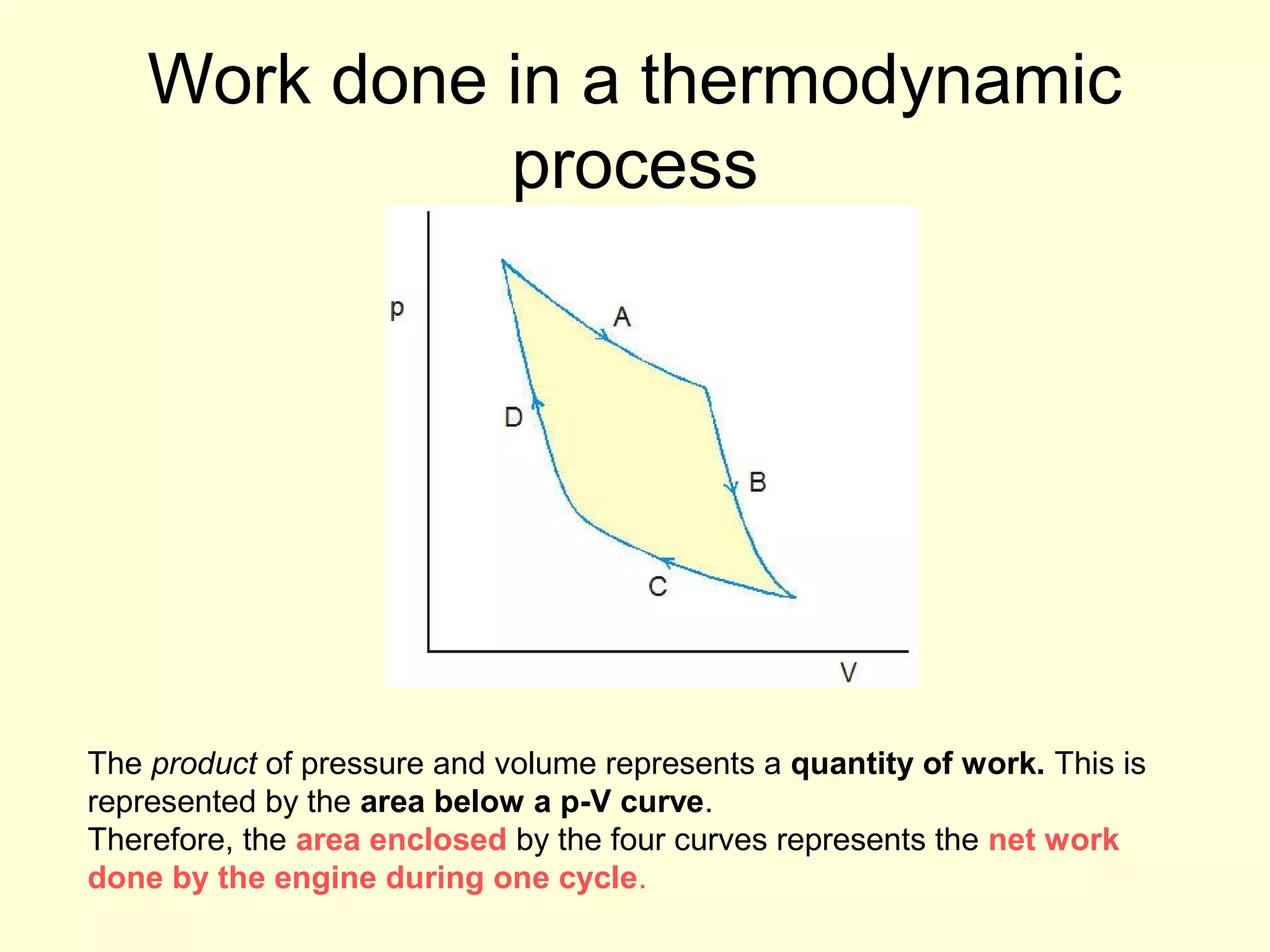
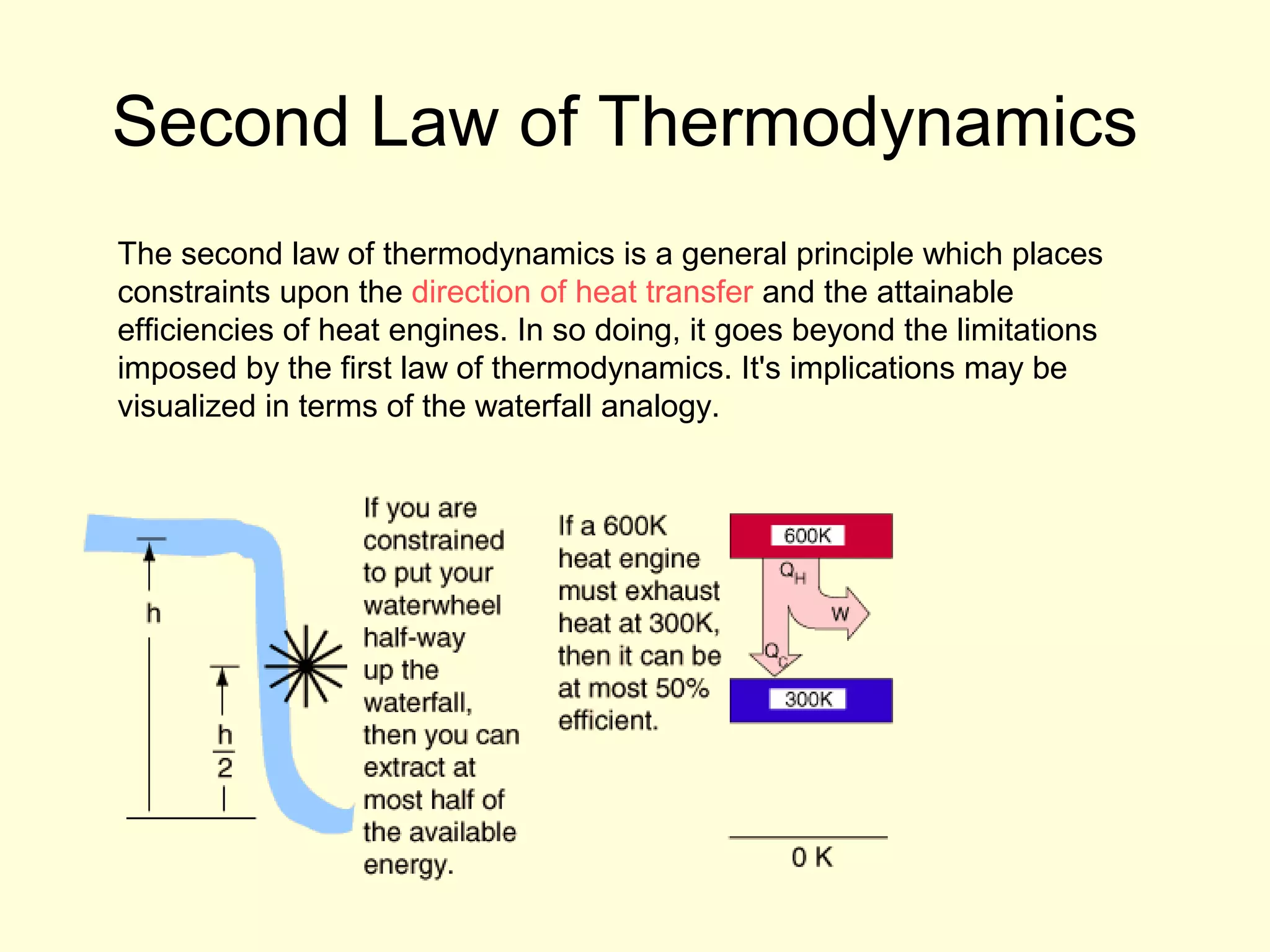
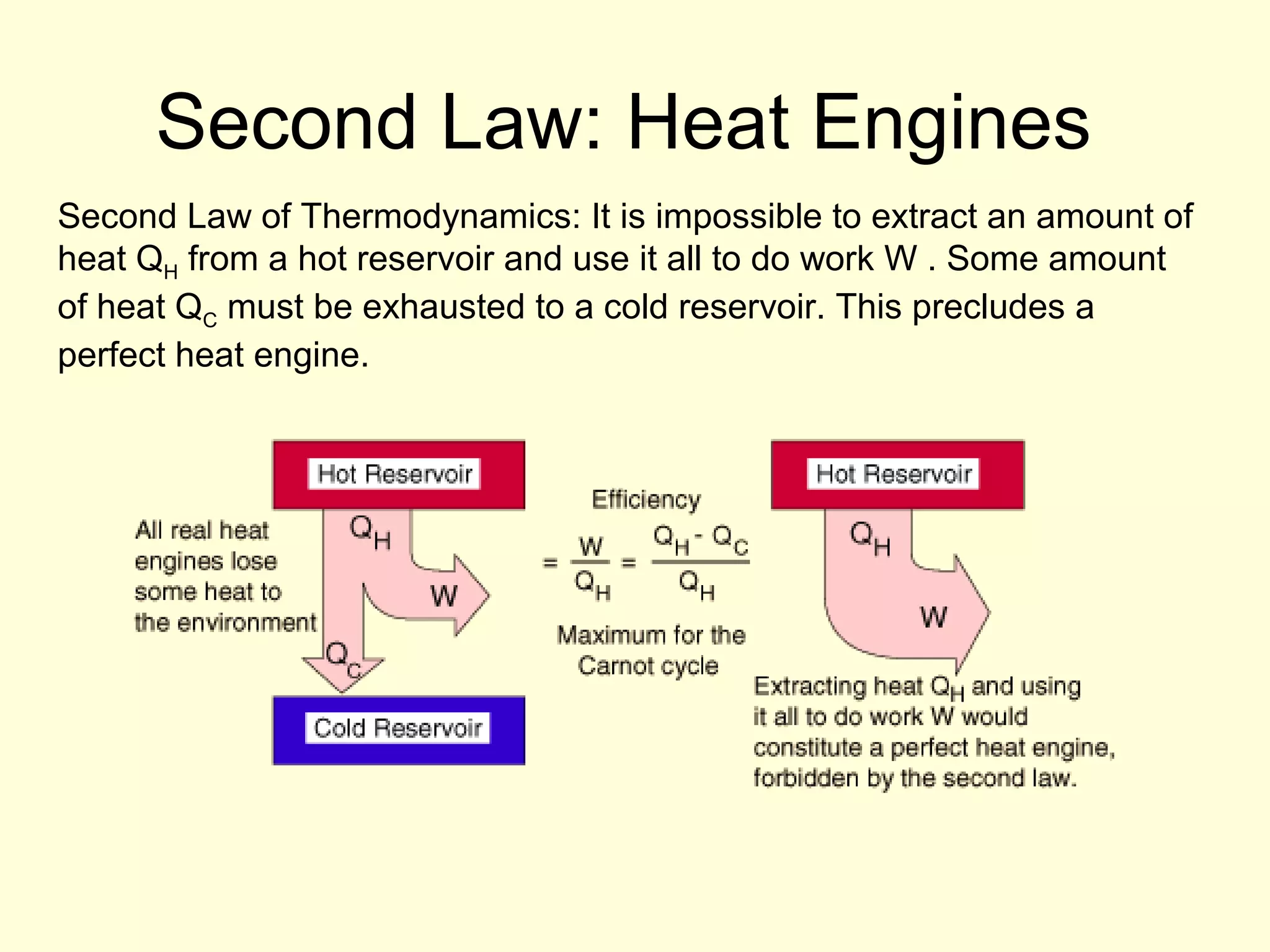
This document discusses the kinetic theory of gases and gas laws. It begins by listing the assumptions of the kinetic theory, including that gas molecules behave as smooth, elastic spheres and are in continuous random motion. It then discusses Gay-Lussac's law relating pressure and temperature, Boyle's law relating pressure and volume, and Charles' law relating volume and temperature. Finally, it introduces the combined gas law and the concept of the ideal gas equation relating pressure, volume, number of moles of gas, and temperature.