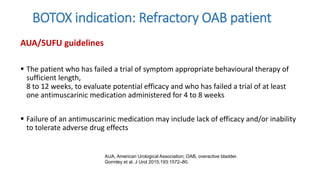
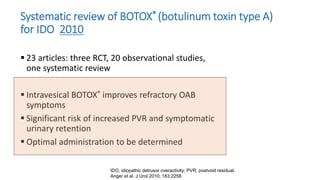
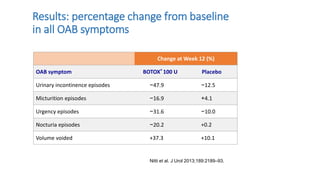
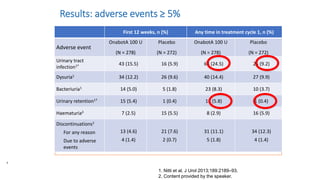
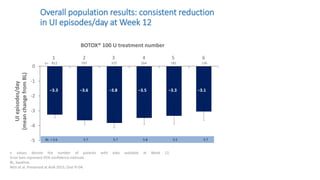
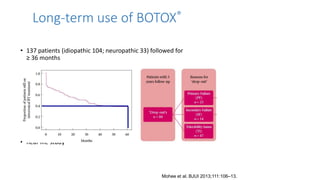
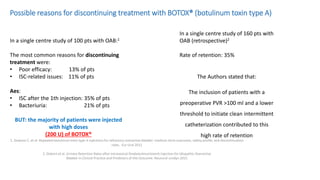
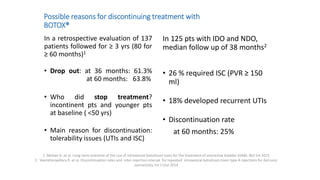
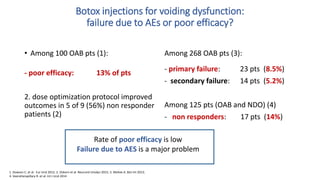
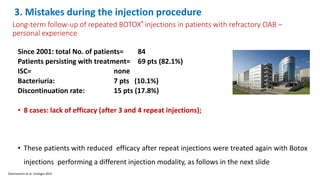
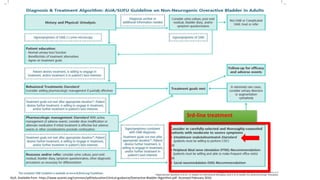
This document discusses the use of botulinum toxin type A (Botox) injections for the treatment of overactive bladder. It provides guidelines from medical organizations on when Botox is an appropriate treatment option. It summarizes several clinical studies that demonstrated the efficacy of Botox in improving overactive bladder symptoms like urinary incontinence and urgency. The studies also showed Botox had manageable side effects like urinary tract infections. However, long-term use of Botox can cause some patients to discontinue treatment due to issues like urinary retention requiring clean intermittent catheterization. The document discusses techniques to optimize outcomes from Botox injections like modifying injection locations and methods.