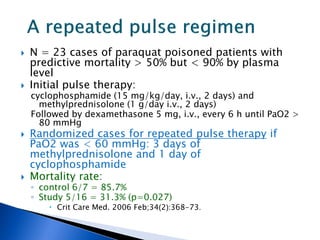
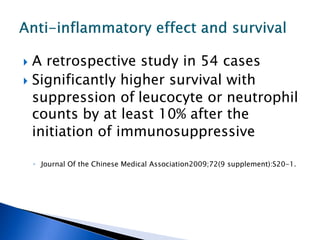
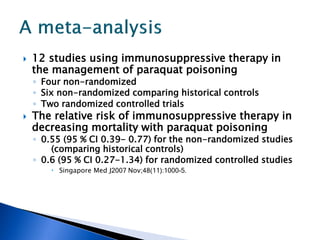
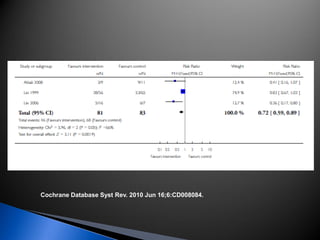
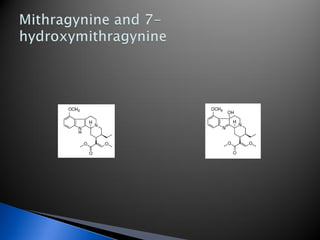
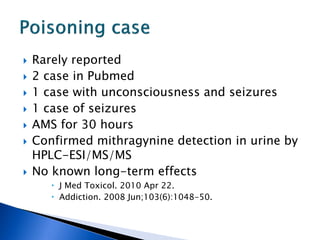
A 55-year-old man was found unconscious at home after ingesting kratom and alcohol. At the emergency department, he was comatose with low vital signs. Treatment with naloxone had no effect. He was given supportive care and woke up 10 hours later, admitting to ingesting kratom and whiskey. Kratom contains compounds that are opioid receptor agonists and can cause respiratory depression, especially in combination with other depressants like alcohol.







































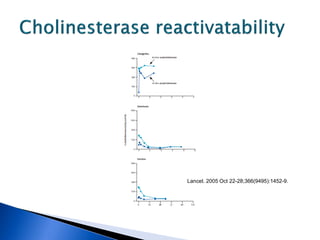
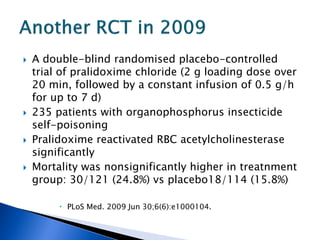
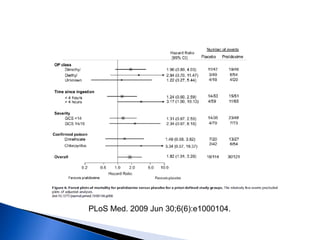
![ A randomized controlled trial studied the effect of
very-high-dose pralidoxime
Pralidoxime iodide (2 g loading dose, then 1 g either
every hour or every 4 h for 48 h, then 1 g every 4 h
until recovery)
200 patients with moderate organophosphorus
poisoning
The high-dose regimen was associated with reduced
case fatality (1% vs 8%; odds ratio [OR] 0·12, 95% CI
0·003–0·90),
Lancet. 2006 Dec 16;368(9553):2136-41.](https://image.slidesharecdn.com/updatetox2010june2010-summonchomchai-100623092650-phpapp01/85/Toxicology-updates-45-320.jpg)