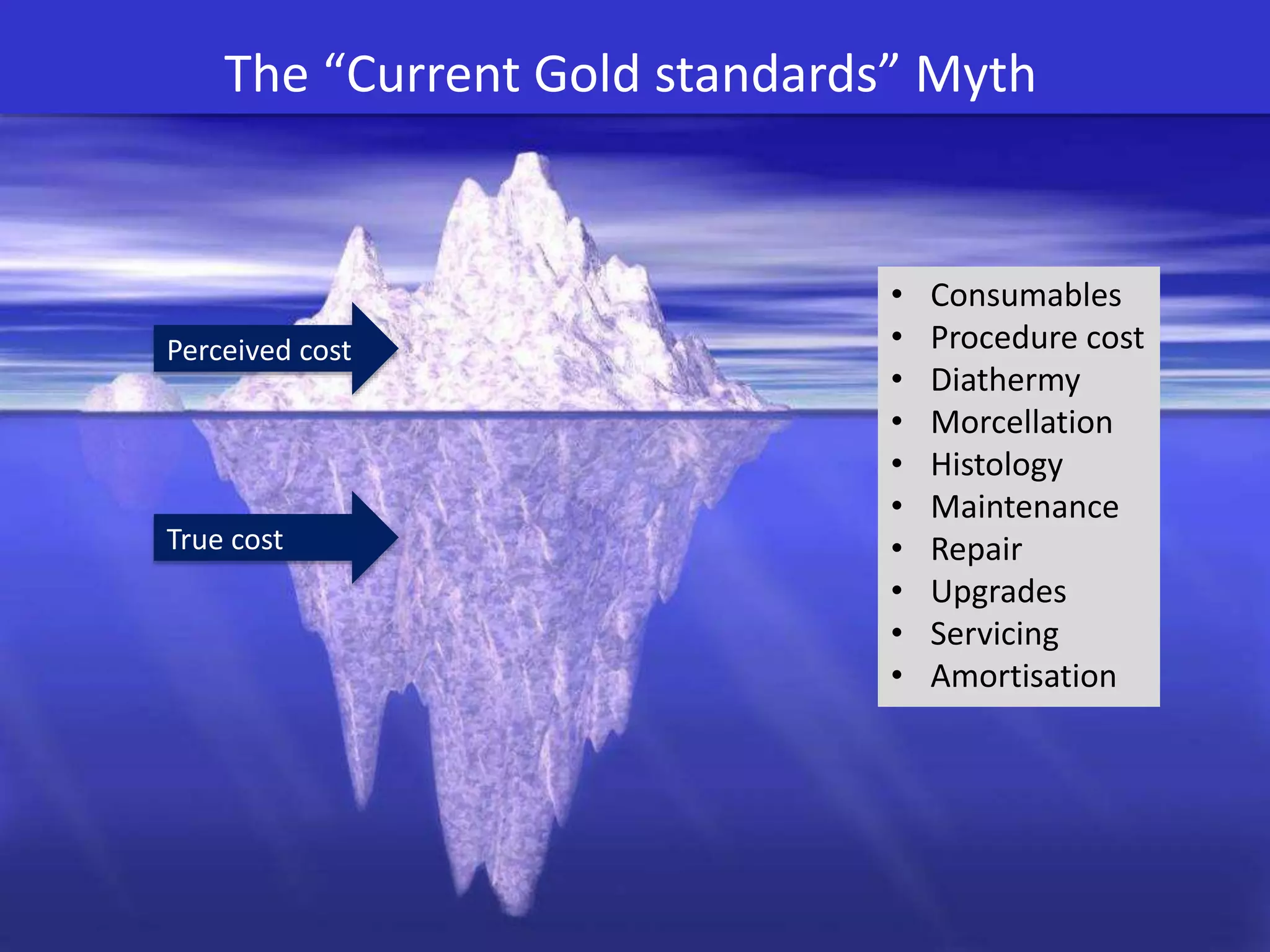
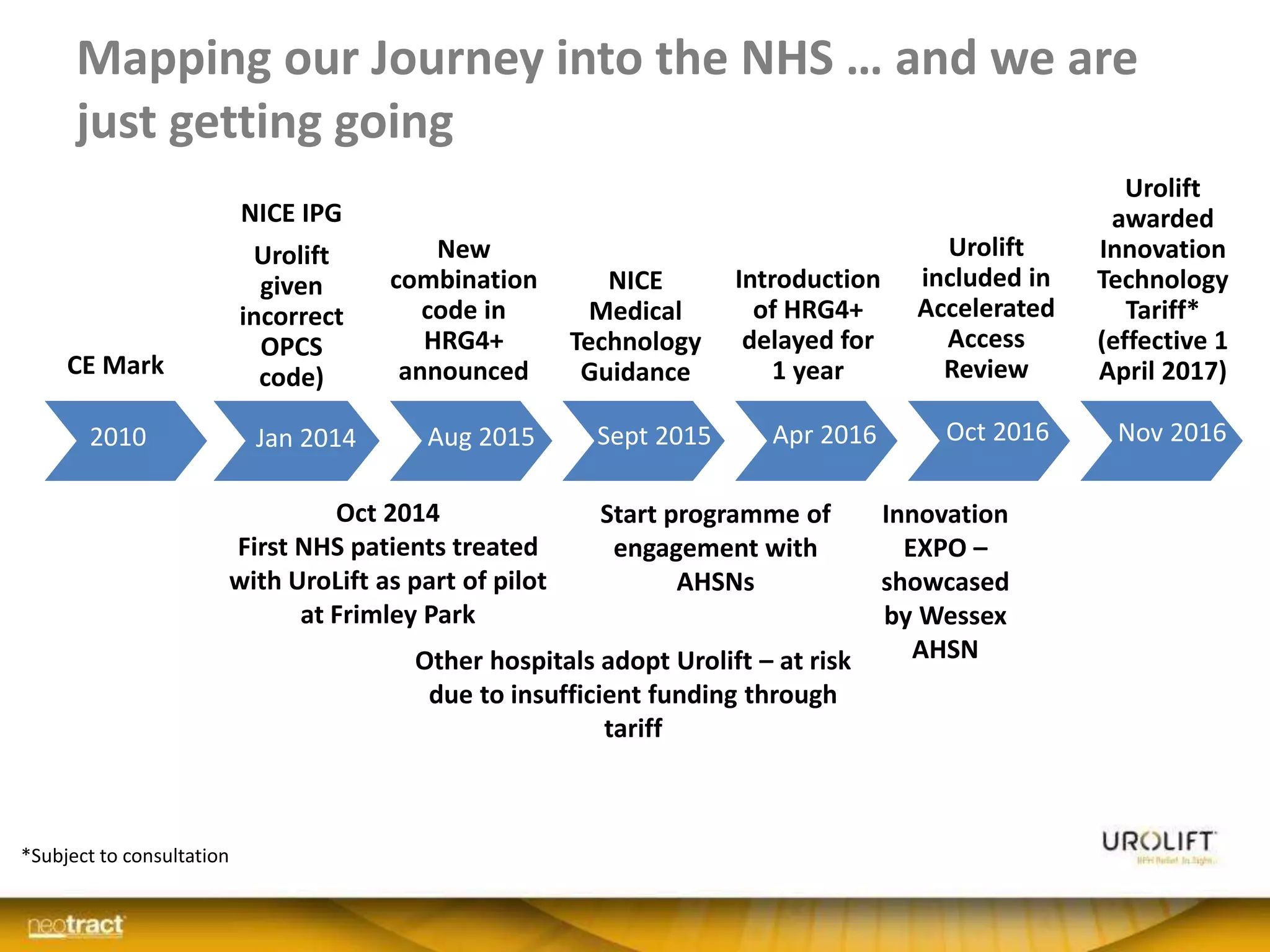
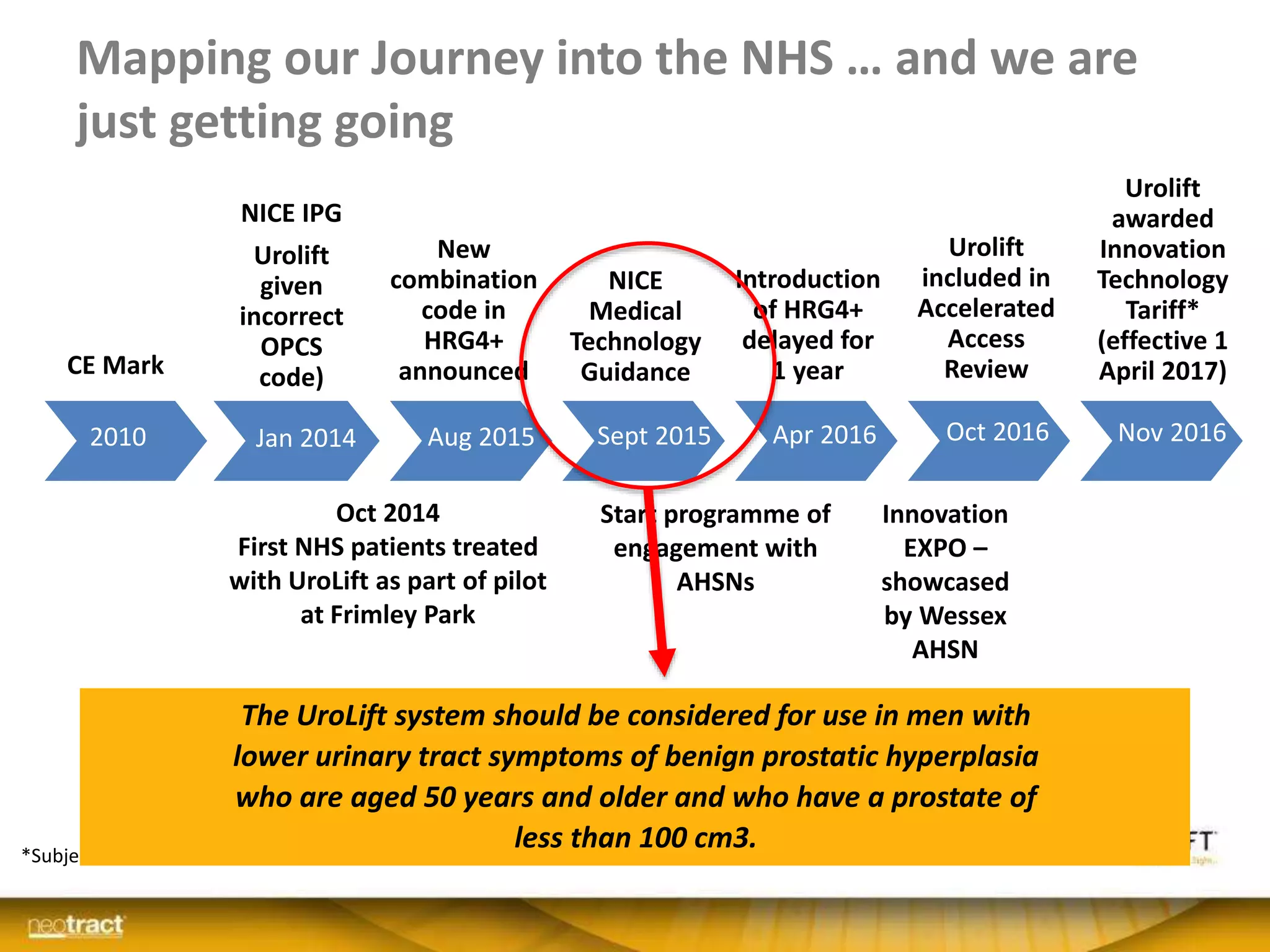
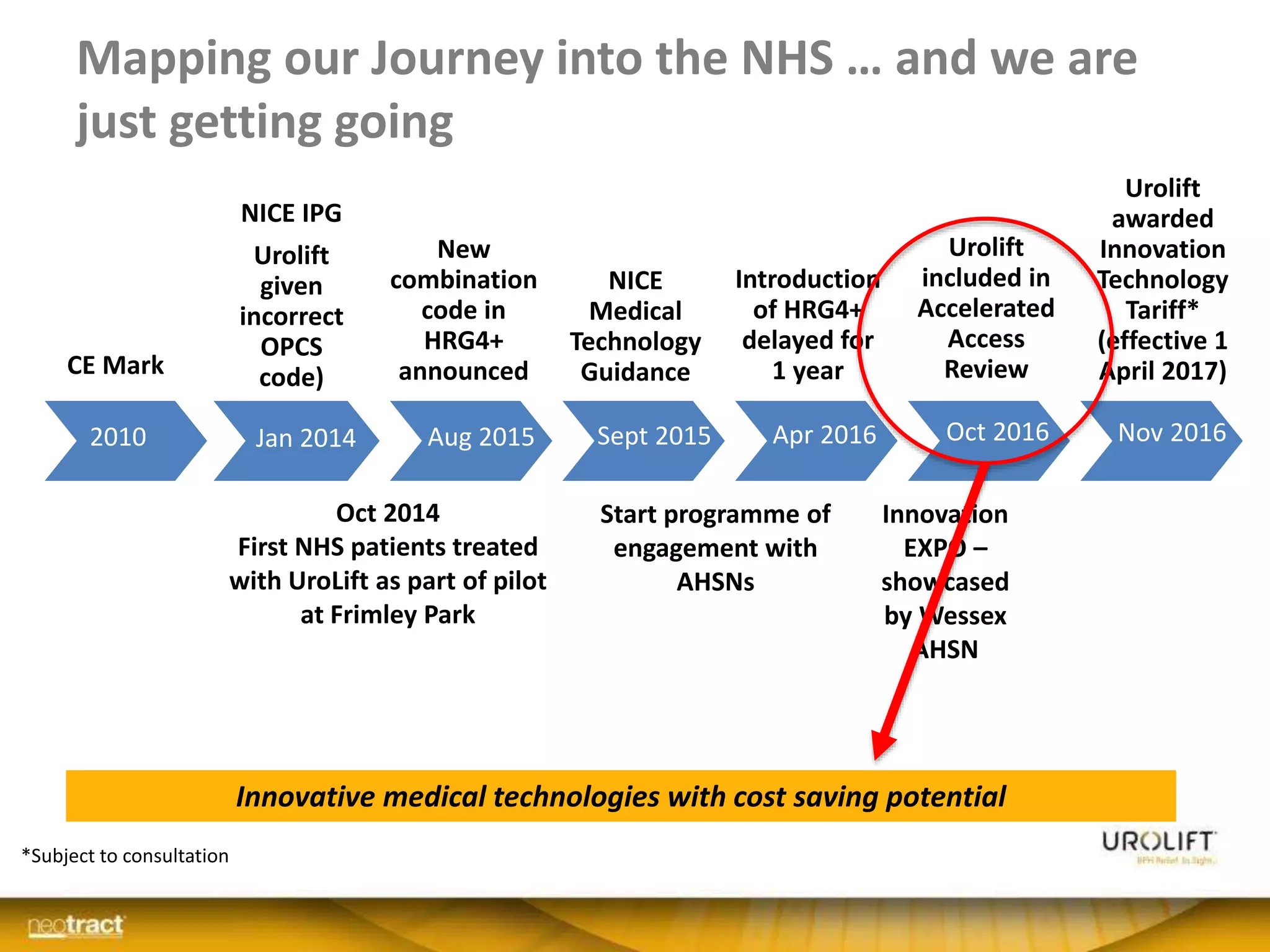
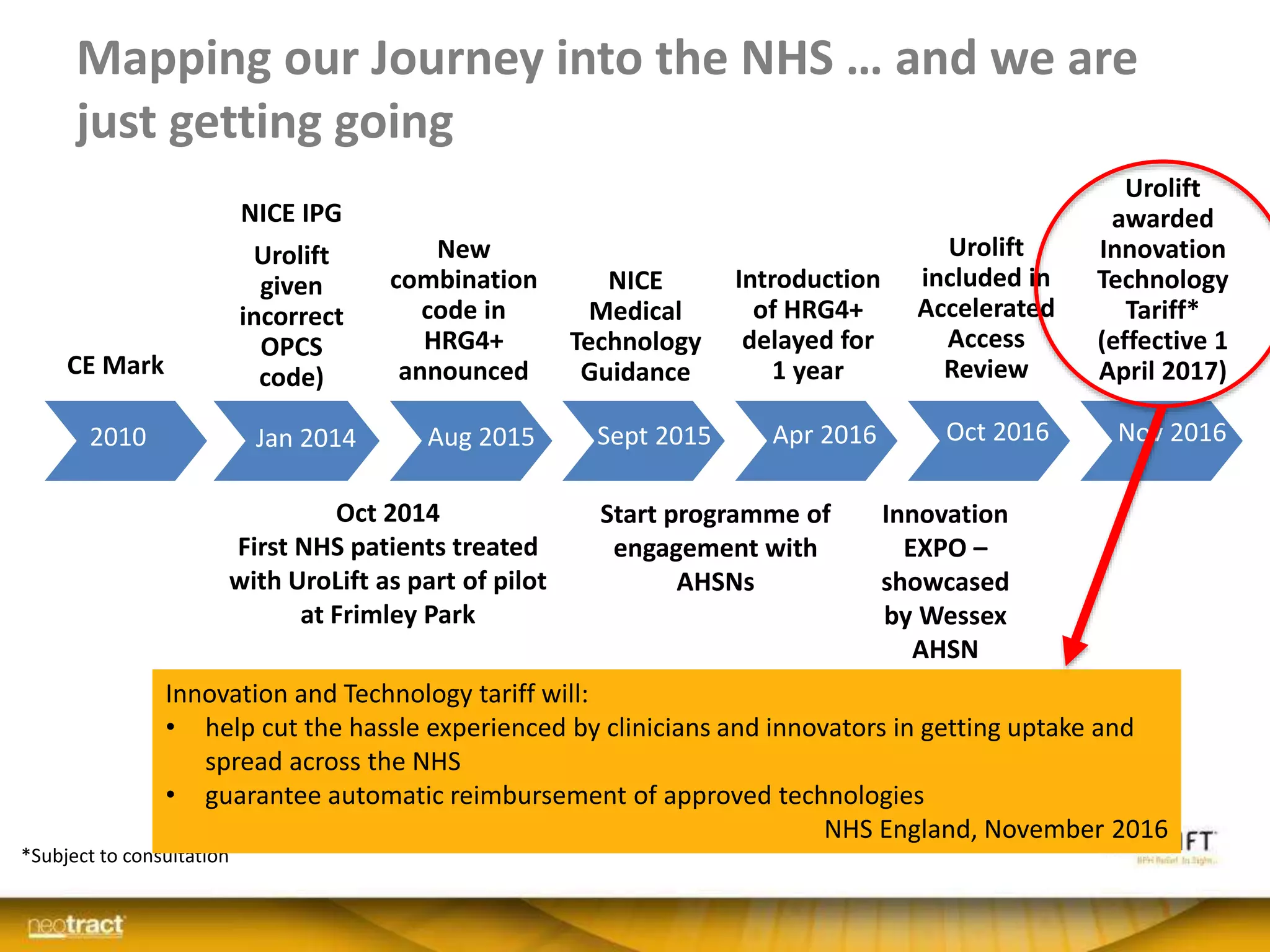
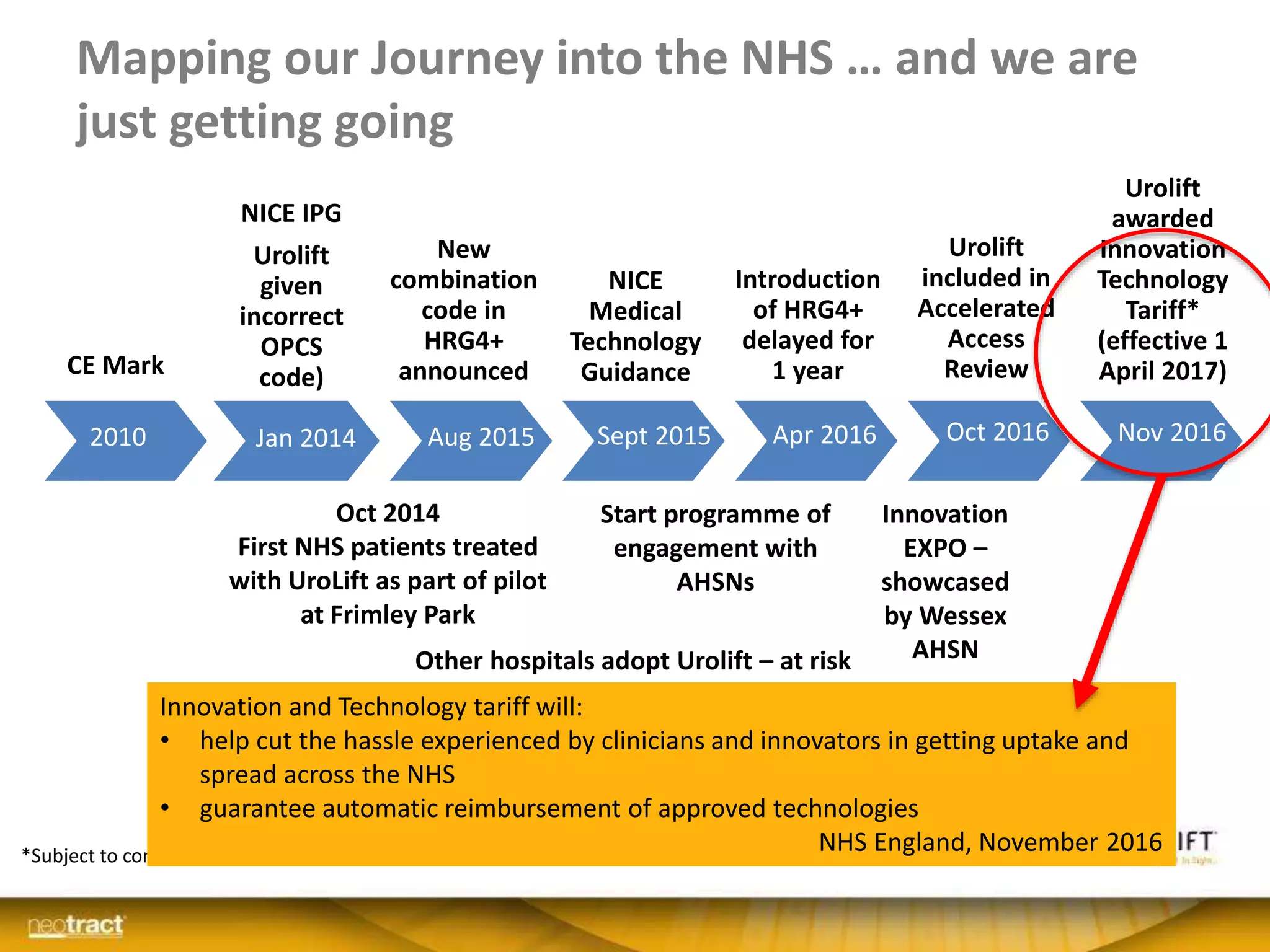
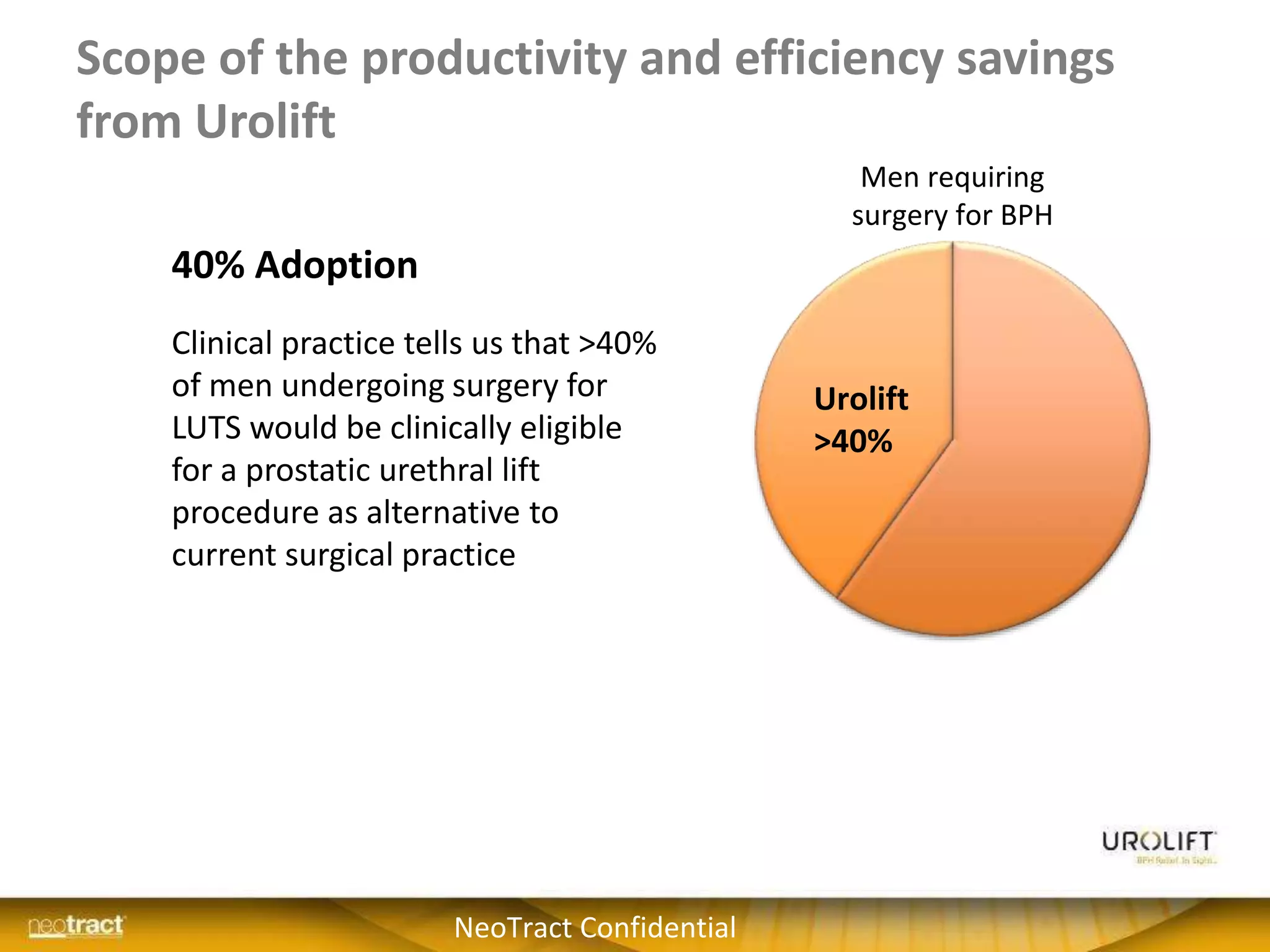
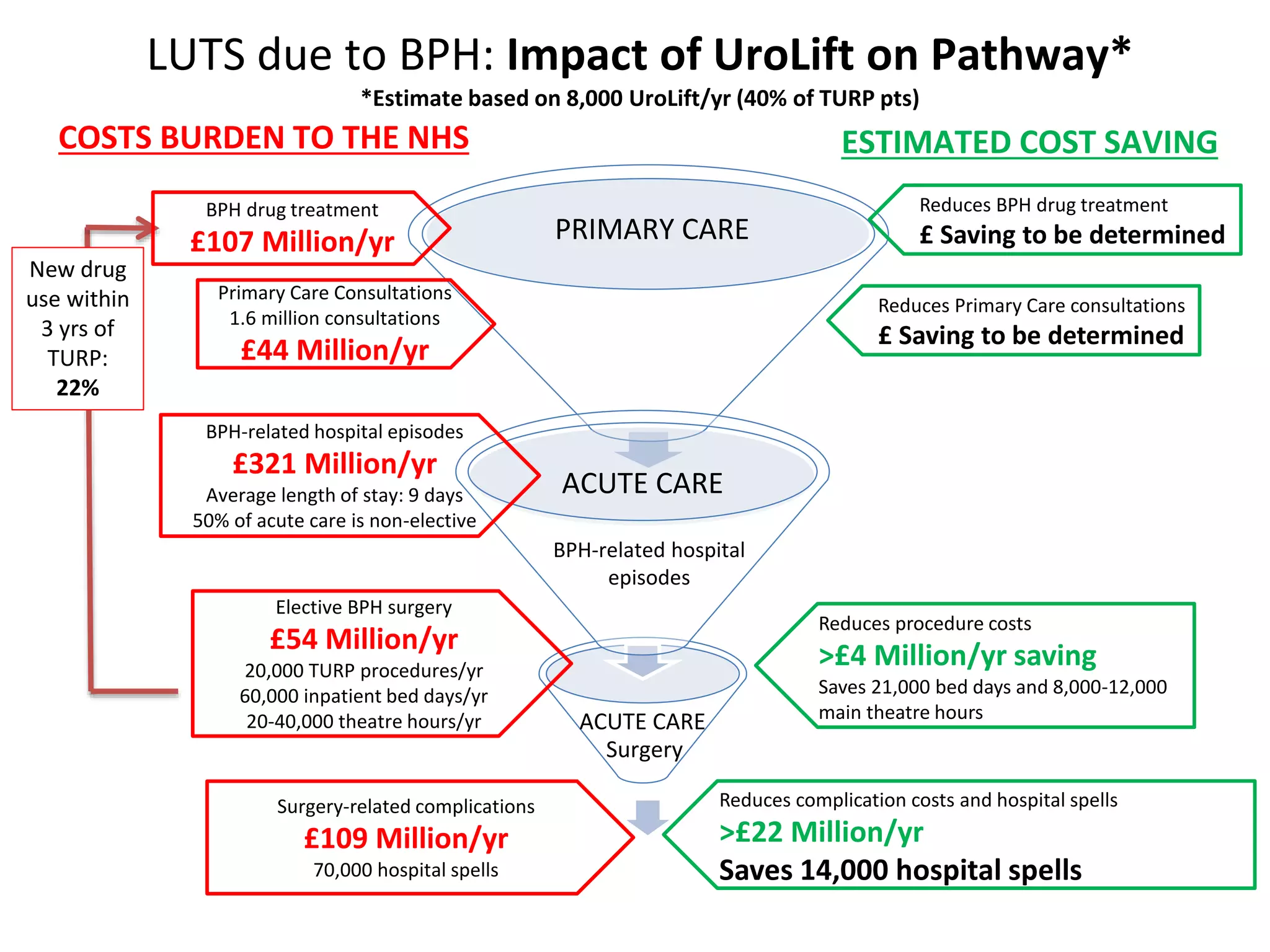
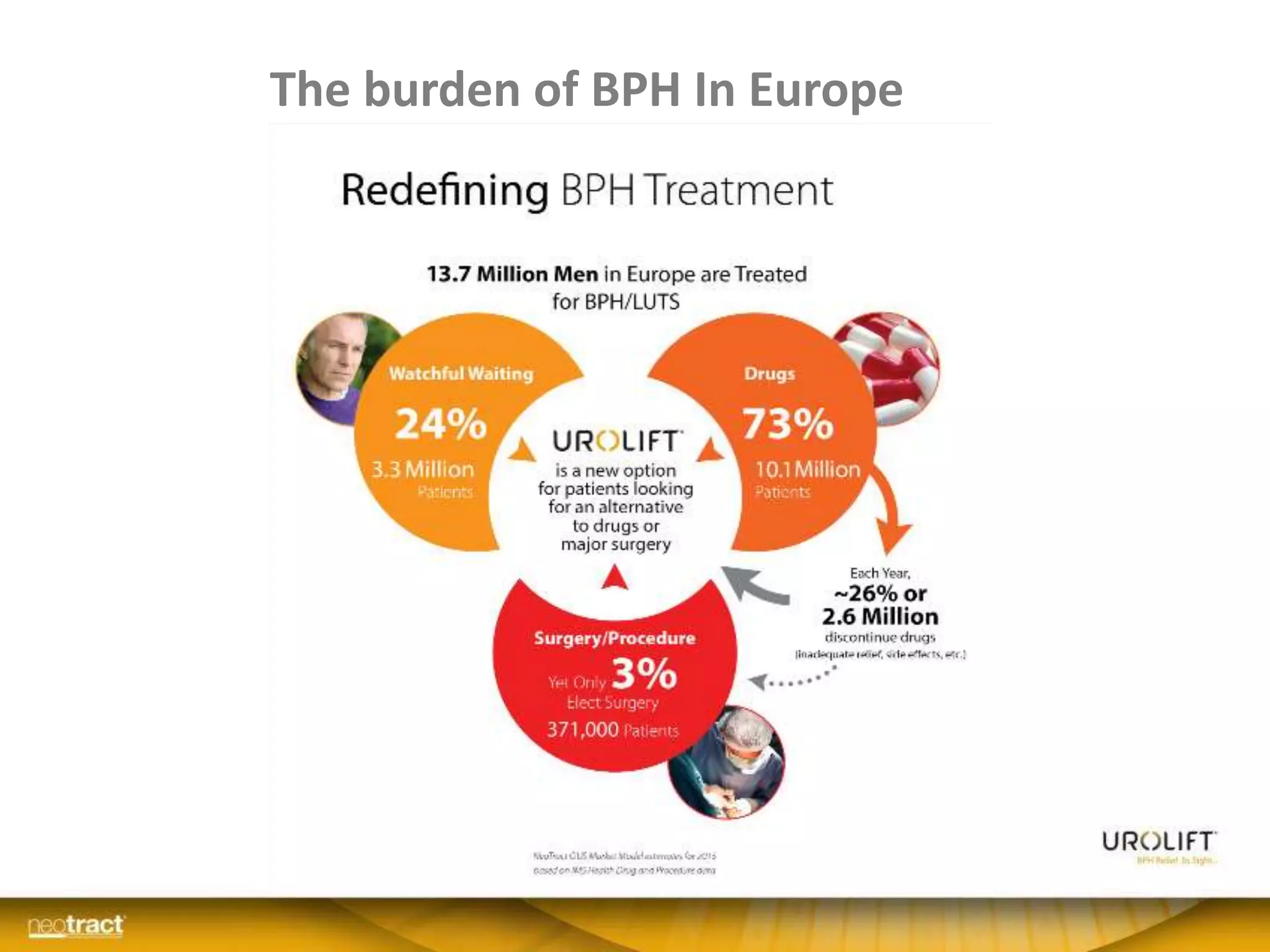
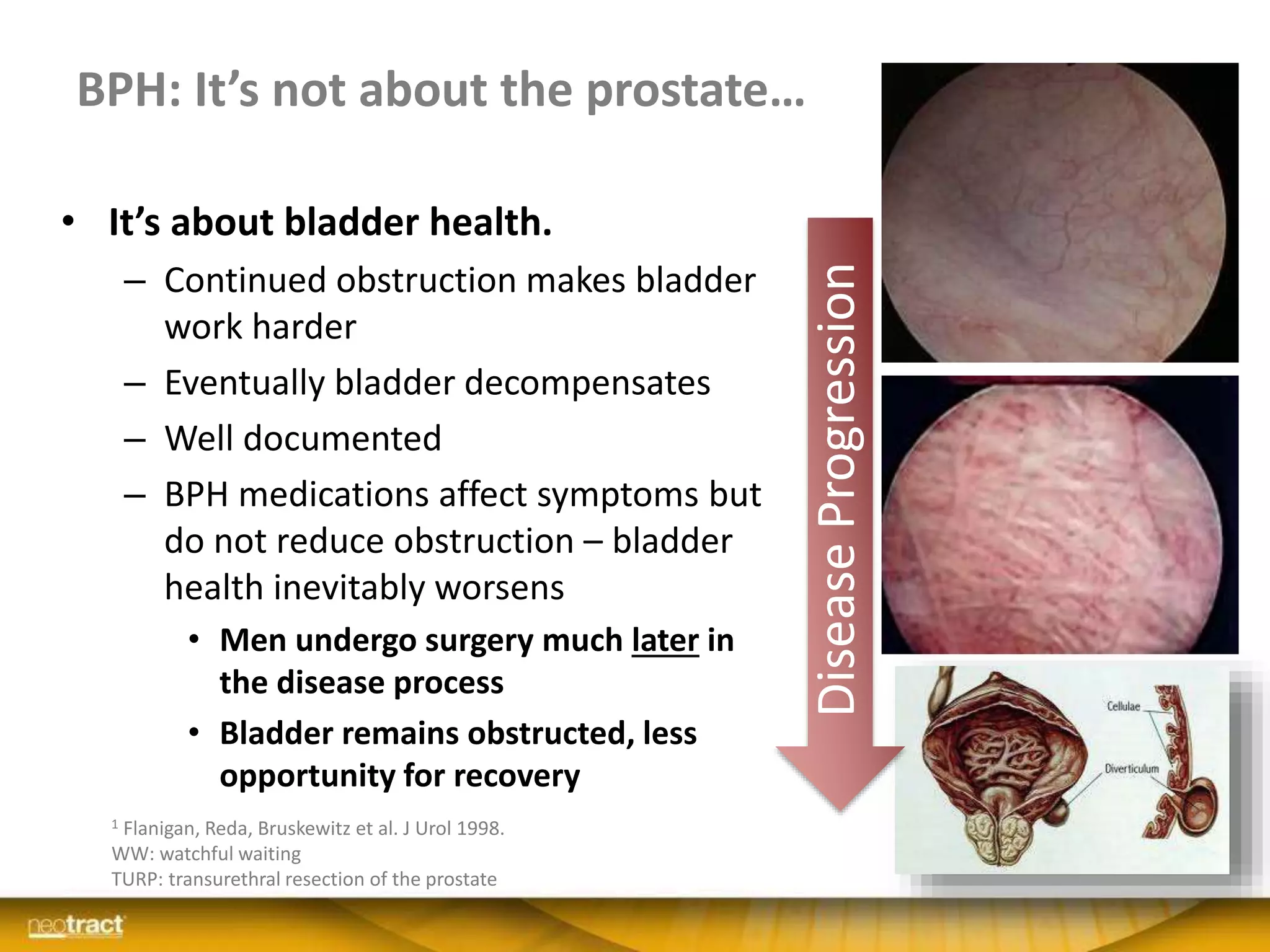
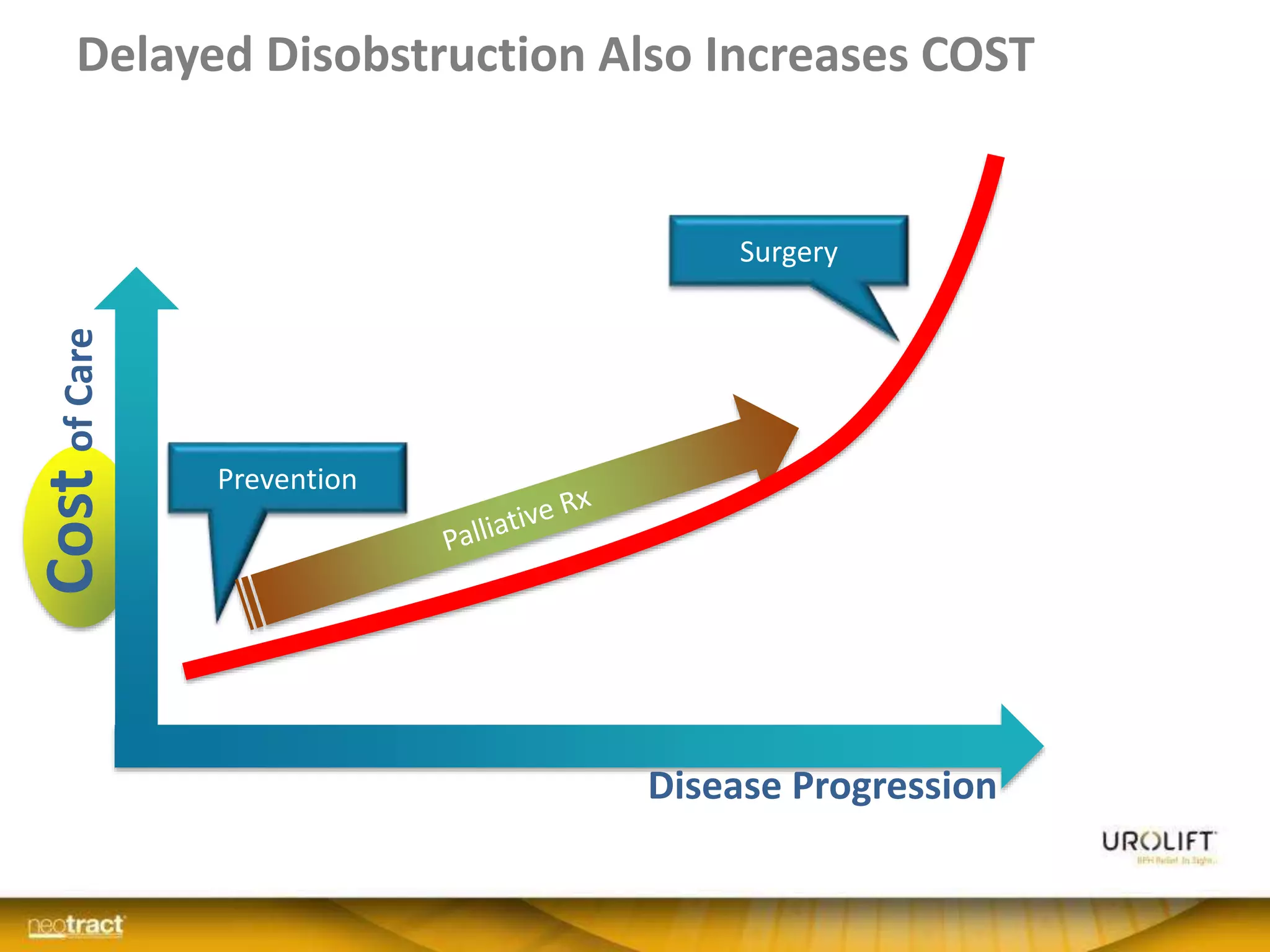
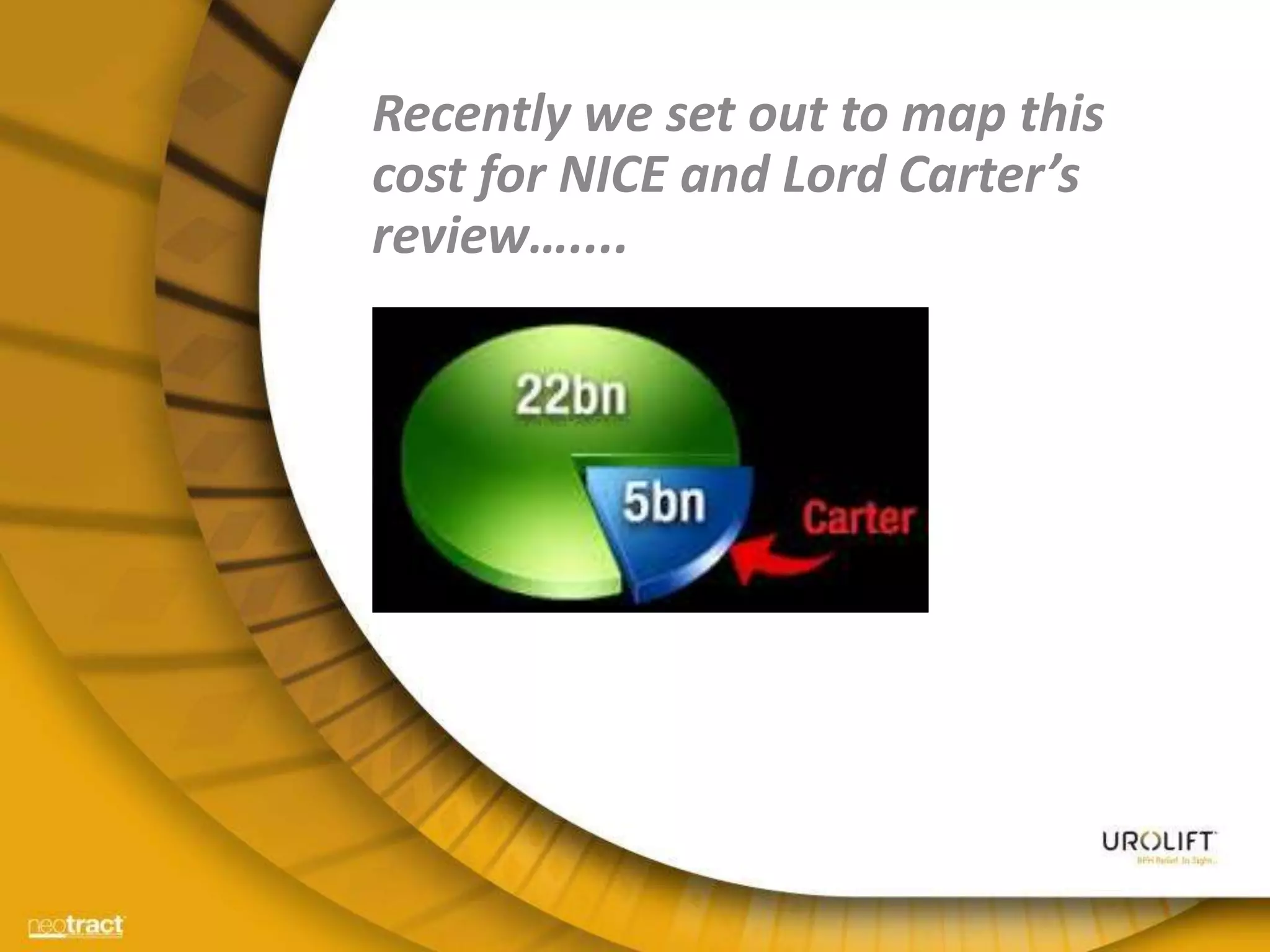
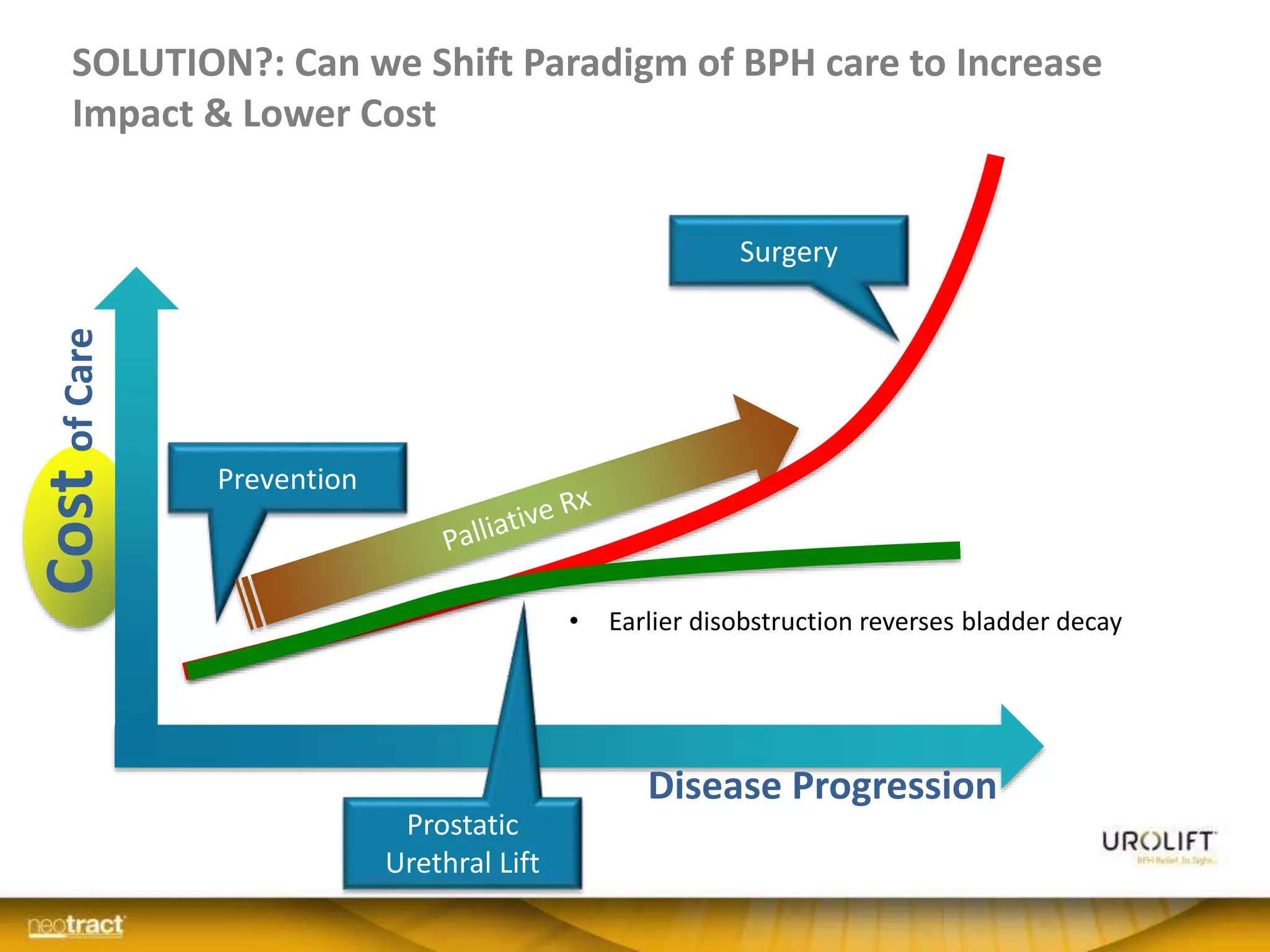
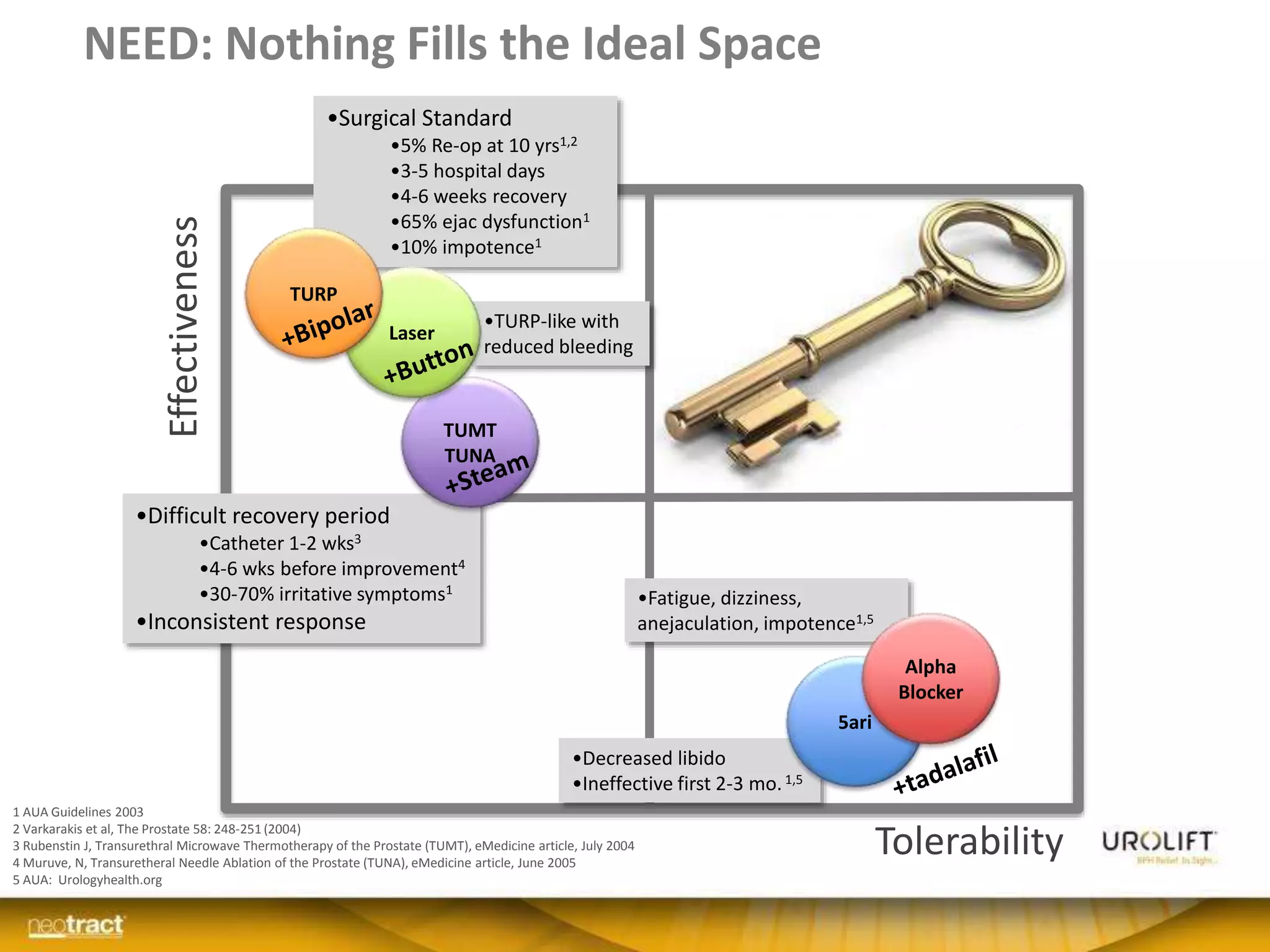
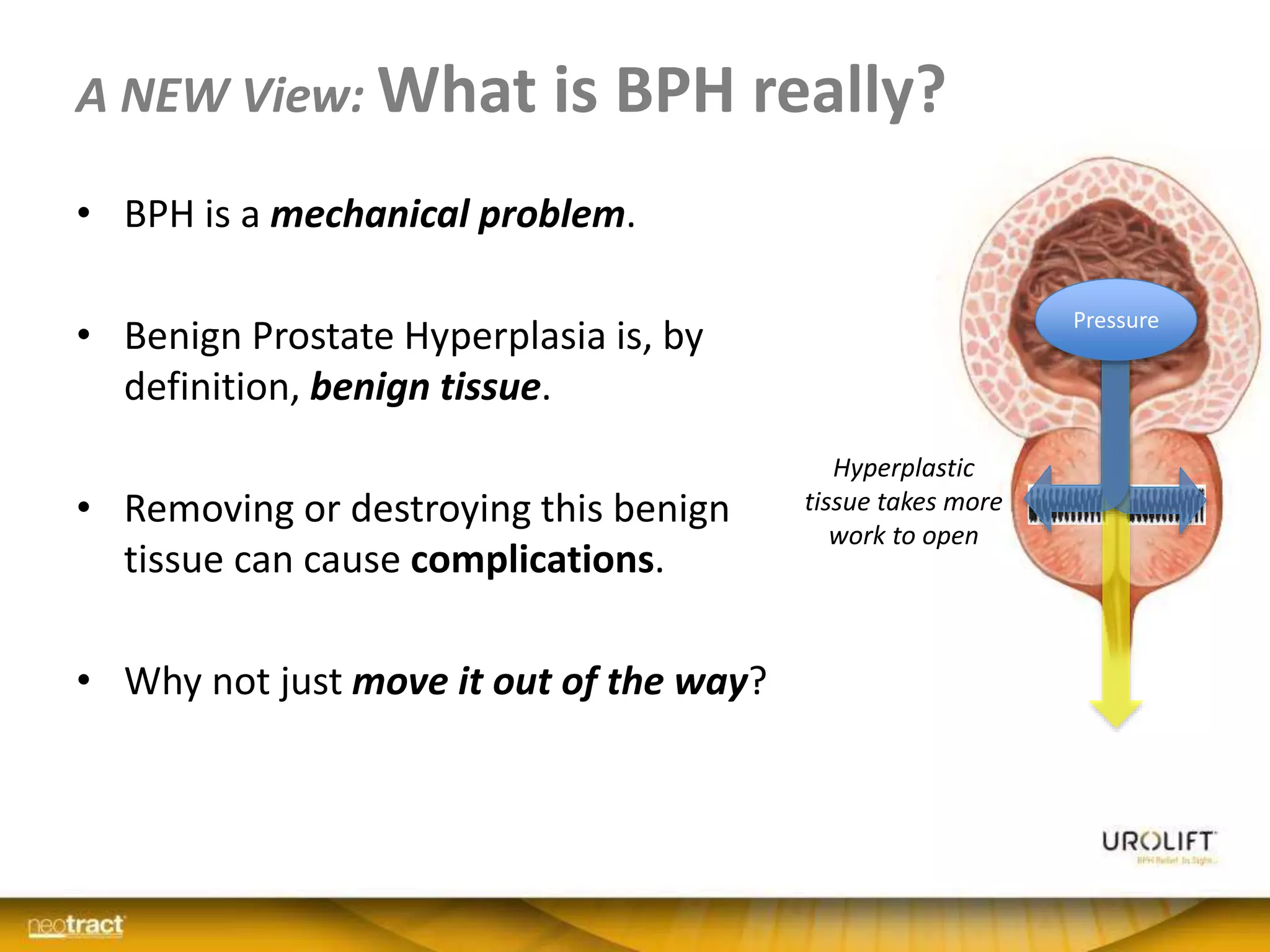
The document discusses the introduction and clinical evidence for the UroLift system, a minimally invasive treatment for benign prostatic hyperplasia (BPH). It summarizes the clinical trials demonstrating UroLift's rapid and durable relief of BPH symptoms with minimal side effects. It also outlines UroLift's journey to approval and reimbursement in the UK, including a positive NICE recommendation and being granted an Innovation Technology Tariff to facilitate adoption in the NHS. UroLift is positioned as a cost-effective alternative to traditional BPH surgeries that allows for quicker recovery and preservation of sexual function.



![Only 3 procedures tried for BPH:
1. Remove prostate tissue
• Enucleation: Simple, HoLEP, Robotic, […Water jet?]
• Resection: TURP, TUIP, Bipolar
• Vaporization: PVP laser, HoLAP, Button
1. Injure/scar/ablate prostate tissue
• Microwave (7), TUNA(3)
• Ethanol, Toxins (4), […Steam?]
1. Open the prostate
• Stents(6)
• Excellent disobstruction
• Serious adverse events
• Lengthy recovery
• Modest disobstruction
• Poor consistency, durability
• Lengthy recovery
• Immediate disobstruction
• Irritation
• Complications requiring removal](https://image.slidesharecdn.com/9-161208141015/75/ECO10-Measuring-the-true-pathway-of-innovation-in-the-NHS-20-2048.jpg)
![UroLift Becoming a Standard of Care
11 years of Diligent Development
PUBLISHED
Randomized
Crossover Study
Positive
Guidance
N.I.C.E.PUBLISHED
2 Year
Durability
De Novo
Approval
HCPCS Coding
Coverage
AETNA
PUBLISHED
Randomized
Blinded Study
PUBLISHED
Sexual
Function
Over 8,000
treated
PUBLISHED
3 Year
Randomized
Durability
PUBLISHED
BPH6 Study:
Randomized
to TURP
Coverage
Medicare 49 states
Kaiser, Aetna
Several Blue Cross
Several privates
PUBLISHED
‘Real-World’
European
Registry
PUBLISHED
Safety &
Feasibility
Category 1
CPT Codes
[Effective Jan’15]
PUBLISHED
LOCAL Study
MAC00226-01 Rev A Positive MTEP
N.I.C.E.](https://image.slidesharecdn.com/9-161208141015/75/ECO10-Measuring-the-true-pathway-of-innovation-in-the-NHS-26-2048.jpg)

![Improved Quality of Care
• UroLift patients recover more quickly
– TURP catches up only between 6 to 12 months
• UroLift patients satisfied sooner and to greater extent
20%
30%
40%
50%
60%
70%
80%
90%
100%
0 3 6 9 12
Recovered(QoRVAS)
Months
PUL
TURP
p<0.05
p<0.05
Sonksen et al. Eur Urol 2015; 68; 643-652.
55%
60%
65%
70%
75%
80%
85%
90%
95%
1 2 3 4 5 6 7 8 9 10 11 12
SatisfiedPatients*
Months
PUL
TURP
*would recommend procedure
PUL randomized to TURP [gold standard surgery]](https://image.slidesharecdn.com/9-161208141015/75/ECO10-Measuring-the-true-pathway-of-innovation-in-the-NHS-30-2048.jpg)