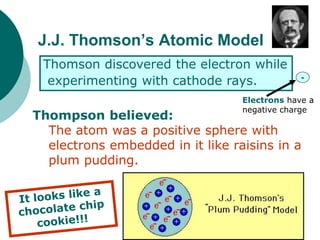
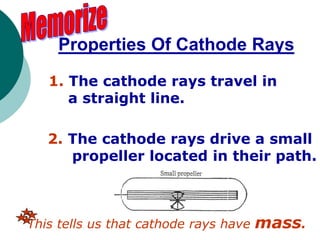
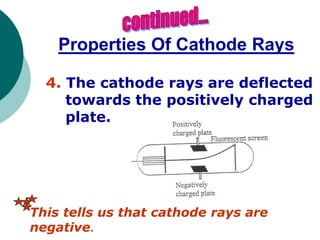
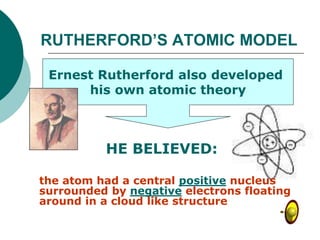
This document discusses J.J. Thomson and Ernest Rutherford's atomic models. It summarizes Thomson's cathode ray experiments that discovered the electron. Thomson believed atoms were a uniform positive sphere with electrons embedded in it. The document also describes Rutherford's model with a small, dense positive nucleus surrounded by electrons in orbit. It lists the key properties of cathode rays that revealed electrons are negatively charged and have mass.