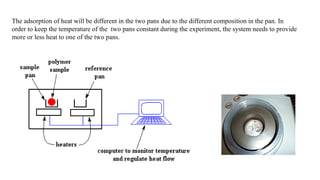
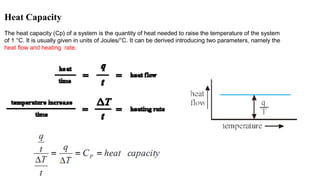
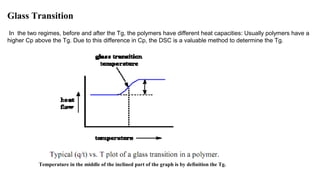
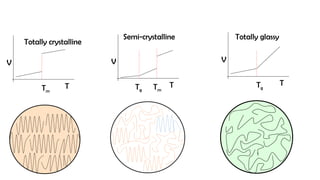
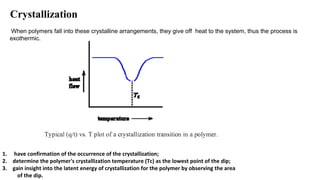
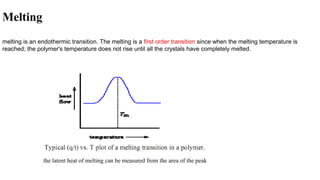
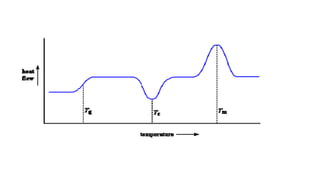
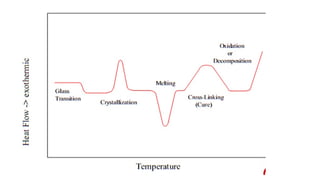
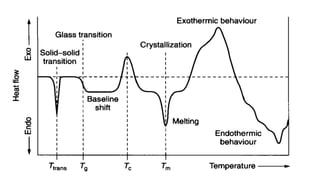
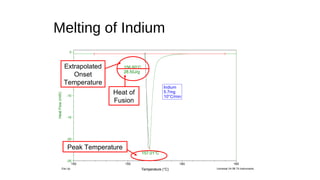
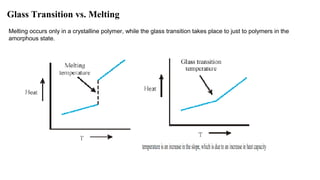
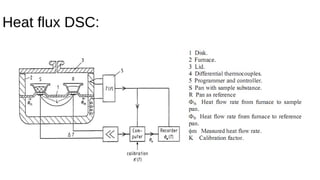
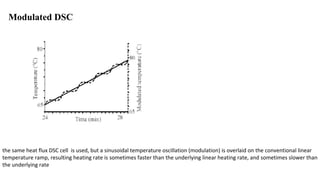
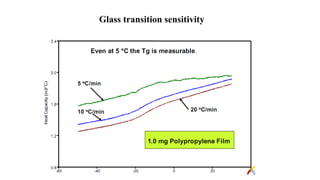
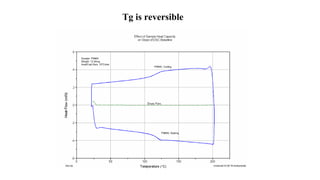
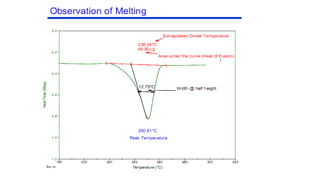
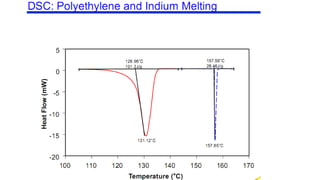
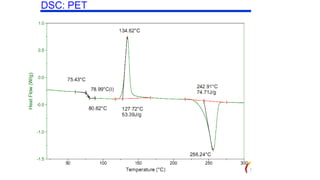
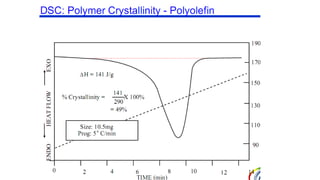
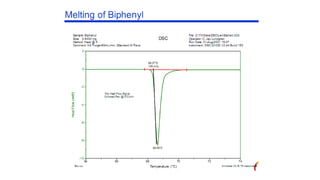
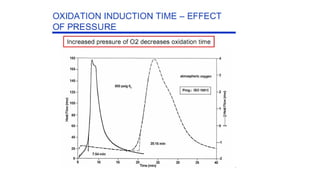
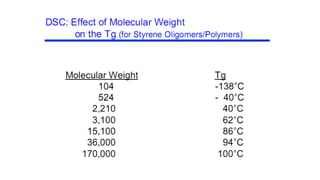
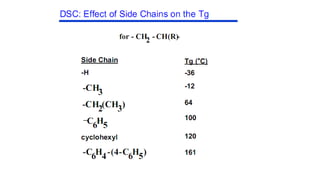
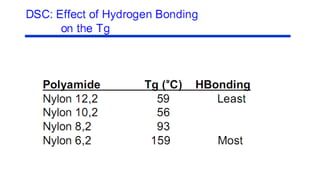
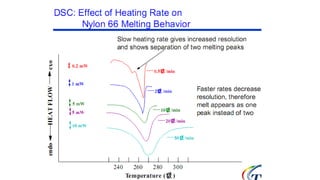
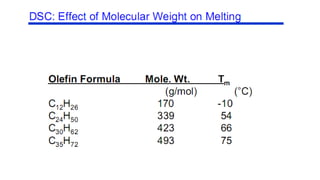
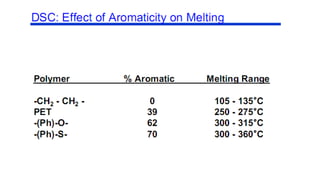
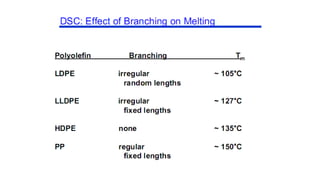
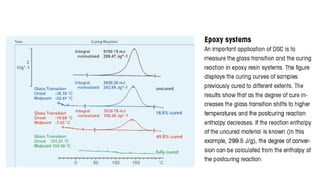
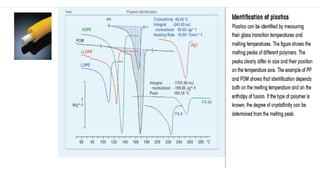
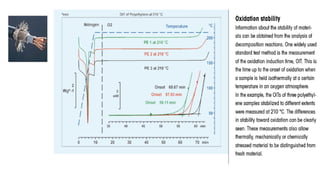
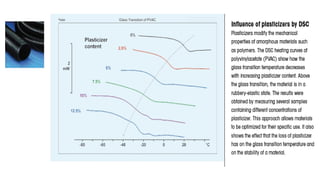
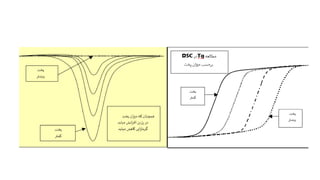
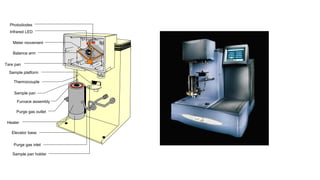
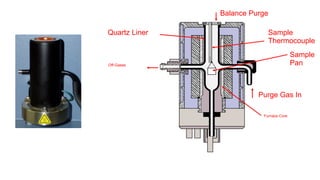
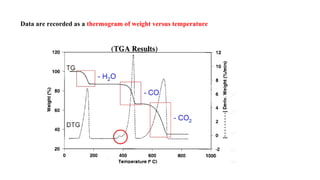
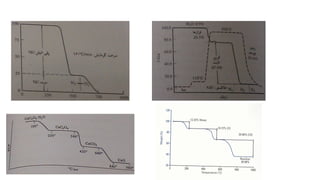
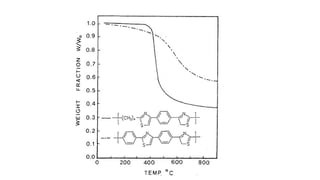
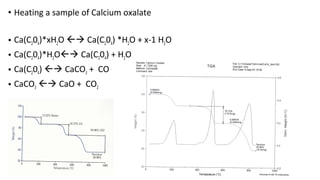
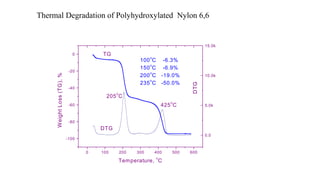
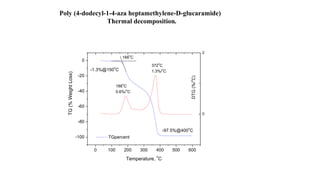
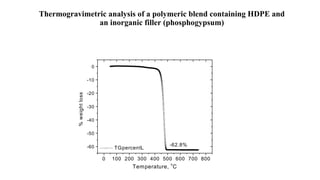
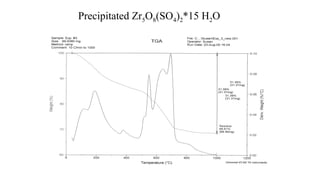
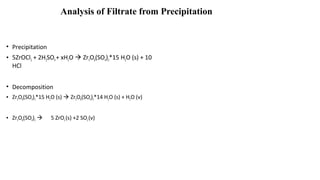
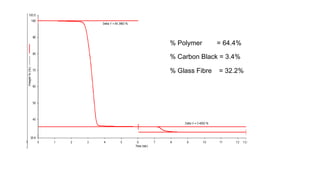
The document discusses differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). DSC measures heat flow during phase transitions and can identify glass transitions, crystallization, and melting. TGA measures weight loss from decomposition as temperature increases. Both techniques provide information on thermal properties like heat capacity, transition temperatures, and decomposition temperatures. Samples are heated at a constant rate while measuring properties like heat flow, weight, or gas evolution to characterize materials.