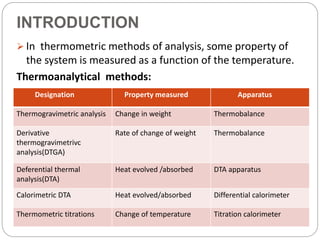
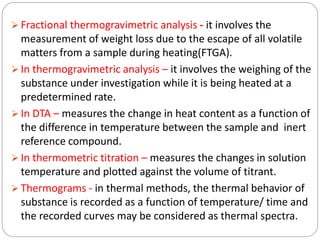
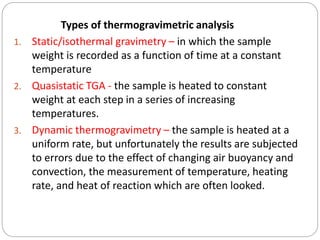
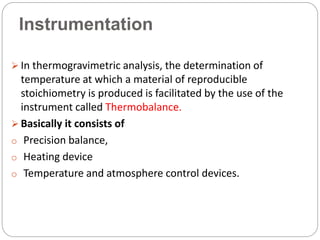
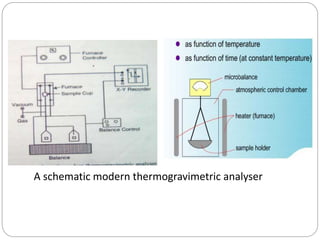
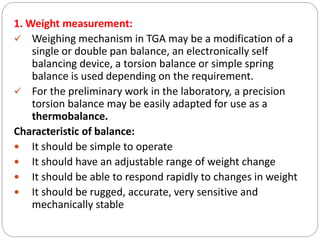
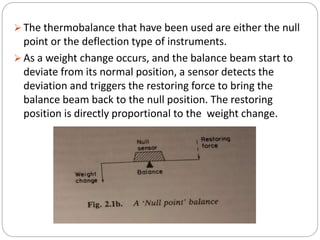
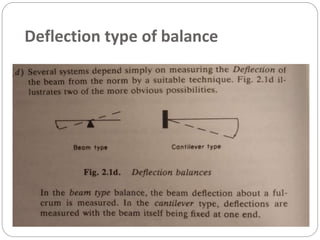
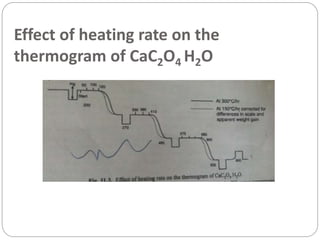
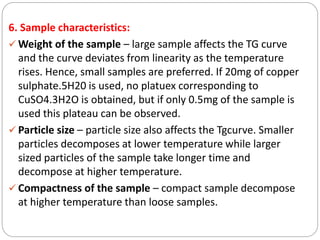
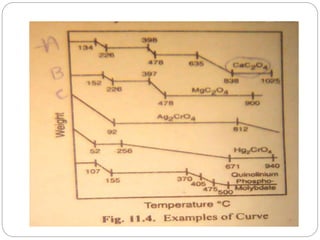
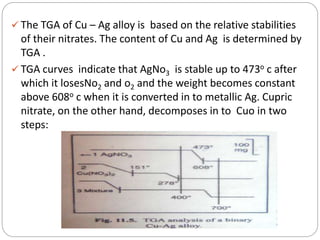
Thermogravimetric analysis (TGA) involves measuring the weight of a substance as it is heated, and can be used to analyze mixtures, determine drying temperatures, and study reaction kinetics. TGA works by heating a sample in a furnace while precisely measuring its weight on a high-precision balance. Derivative thermogravimetric analysis (DTGA) measures the rate of weight change during heating. TGA can identify different components in a mixture based on their unique thermal decomposition profiles, and determine optimum drying ranges to isolate compounds like calcium oxalate. Kinetic parameters of reactions can also be extracted from TGA or DTGA curves using dynamic or isothermal methods.