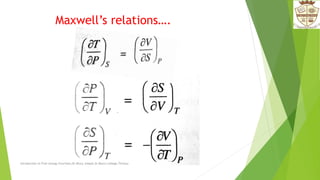
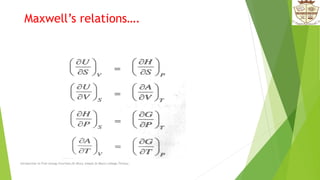
The document introduces free energy functions such as Helmholtz energy and Gibbs free energy. It discusses how these functions can be used to express spontaneity criteria for systems under different constraints of temperature and volume or pressure. Specifically, it describes how Helmholtz energy applies for constant temperature and volume, while Gibbs free energy applies for constant temperature and pressure. The document also examines physical interpretations and relationships between the different free energy functions and how they vary with respect to temperature, volume, and pressure.









![The Gibbs Helmholtz equation…..
TdS = dU + PdV
dG= TdS + VdP – TdS - SdT
dG= VdP - SdT
At constant P, dP=?
dG = -SdT
This can be represented as
[
𝜕𝐺
𝜕𝑇
]p = -S
Introduction to Free energy Functions,Dr.Bincy Joseph,St.Mary's college,Thrissur.](https://image.slidesharecdn.com/introductiontofreeenergyfunctions-200909085755/85/Introduction-to-free-energy-functions-10-320.jpg)
![The Gibbs Helmholtz equation…..
For initial state [
𝜕𝐺1
𝜕𝑇
]p= -S1
For final state [
𝜕𝐺2
𝜕𝑇
]p= -S2
[
𝜕𝐺2
𝜕𝑇
]p - [
𝜕𝐺1
𝜕𝑇
]p= - (S2 –S1)
[
𝜕(𝐺2−𝐺1)
𝜕𝑇
]p = -ΔS
[
𝜕(Δ𝐺)
𝜕𝑇
]p = -ΔS
ΔG = ΔH-TΔS
ΔG = ΔH + T [
𝜕(Δ𝐺)
𝜕𝑇
]p
Introduction to Free energy Functions,Dr.Bincy Joseph,St.Mary's college,Thrissur.](https://image.slidesharecdn.com/introductiontofreeenergyfunctions-200909085755/85/Introduction-to-free-energy-functions-11-320.jpg)