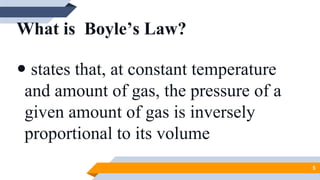
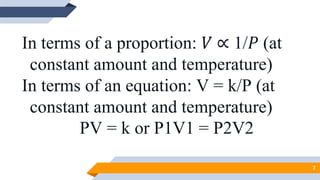
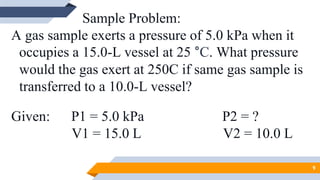
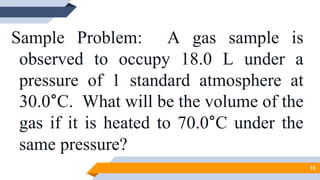
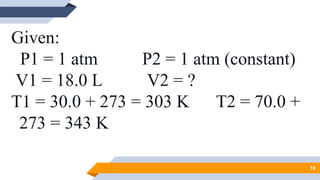
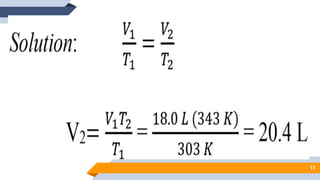
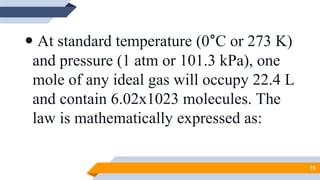
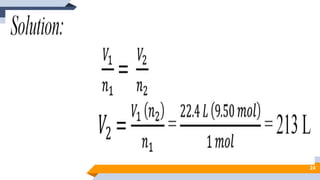
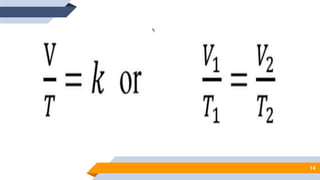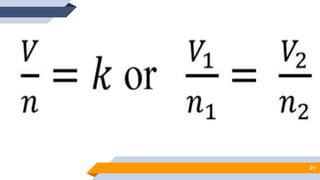The document discusses the ideal gas laws, including Boyle's law, Charles' law, and Avogadro's law. It provides explanations of each law, mathematical expressions of the relationships between variables like pressure, volume, temperature and moles of gas. Examples are given to demonstrate how to use the gas laws to solve problems involving changes in pressure, volume or temperature of an ideal gas. Real-world applications are described for each gas law, such as in lungs, car engines, spray cans and baking.