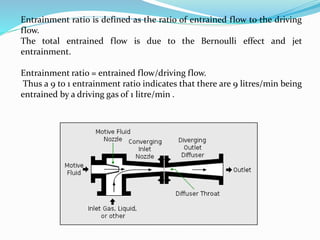
The document discusses various gas laws and their applications in anesthesia and respiratory physiology. It begins by using Boyle's law to calculate the volume of oxygen remaining in a cylinder at a pressure of 15 psig. It then explains Charles, Gay-Lussac's, Avogadro's, Dalton's laws and their relevance. Further sections cover Hagen-Poiseuille's law, Reynolds number, Graham's law, Bernoulli's principle, Venturi effect, Coanda effect, critical temperature, Poynting effect, adiabatic changes, and other gas laws and their importance in areas like gas delivery, flow dynamics, and equipment function.