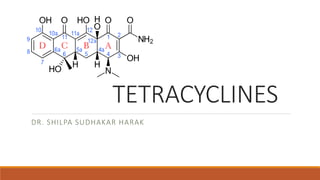
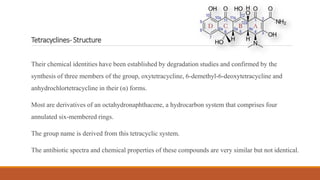
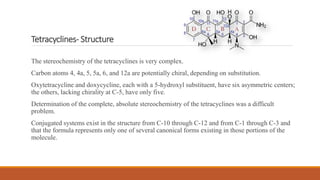
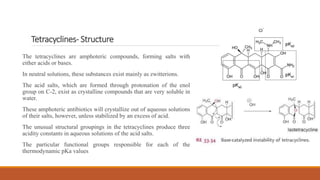
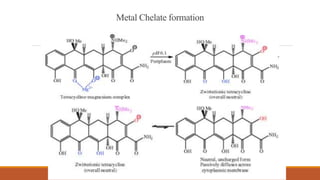
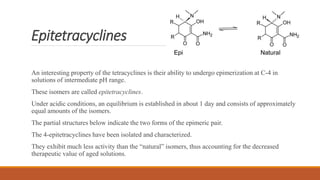
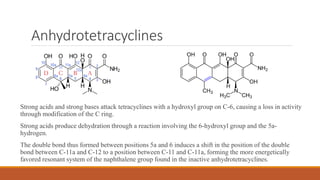
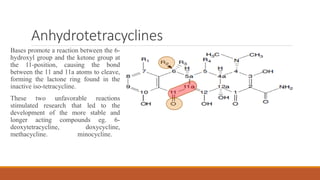
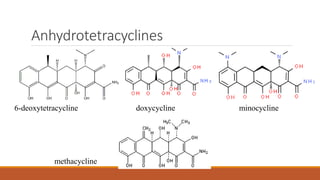
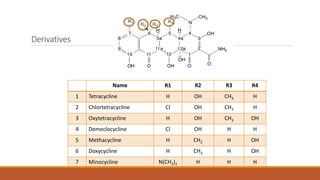
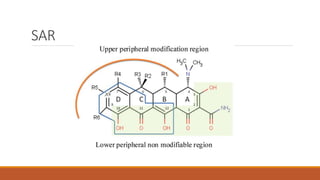
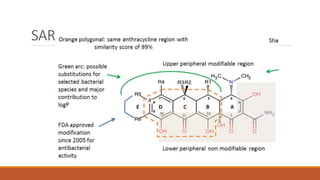
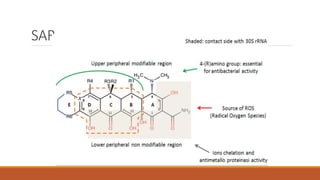
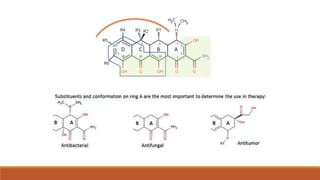
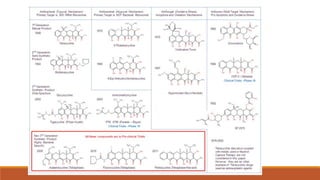
This document discusses tetracycline antibiotics. It describes their structure, mechanism of action, derivatives, and spectrum of activity. Tetracyclines are broad-spectrum antibiotics obtained from Streptomyces bacteria. They work by inhibiting bacterial protein synthesis by binding to the 30S ribosomal subunit. There are several medically used tetracycline compounds that differ slightly in their chemical structures. Resistance can occur through efflux pumps, ribosomal protection, or enzymatic oxidation. The tetracyclines demonstrate the broadest antibacterial spectrum of any antibiotic class.