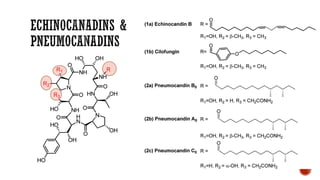
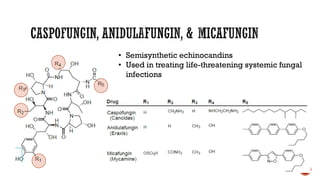
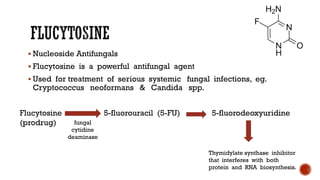
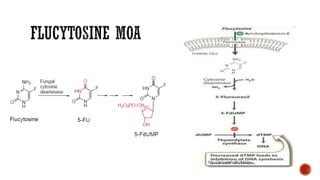
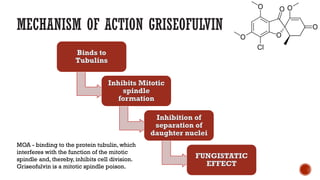
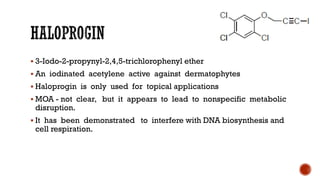
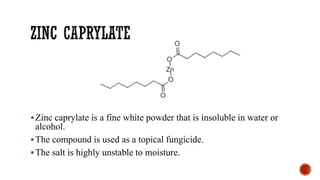
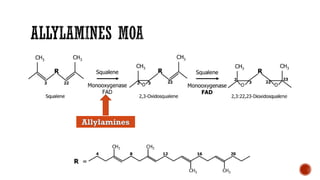
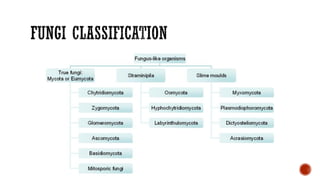
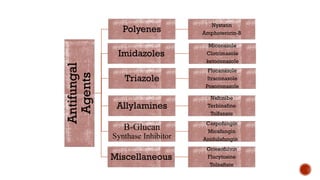
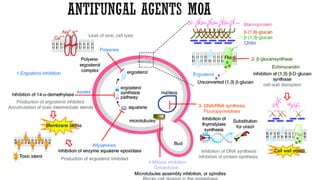
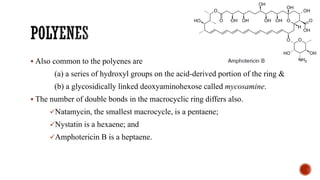
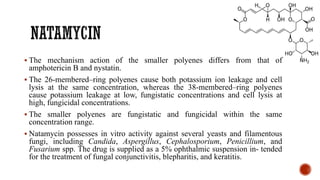
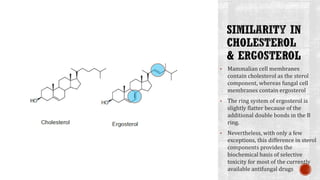
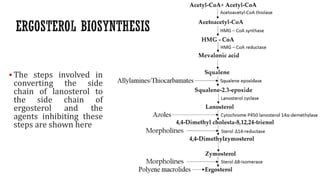
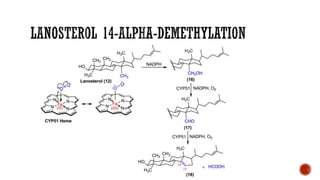
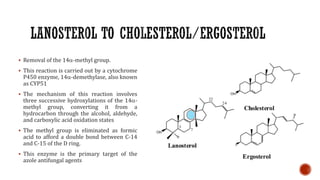
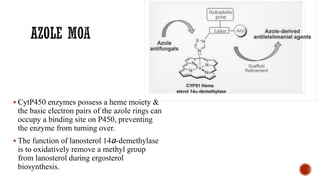
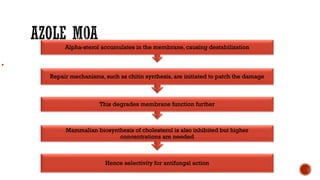
The document discusses antifungal agents, specifically focusing on the mechanisms, classifications, and examples of major antifungal drugs. It details the characteristics of fungal infections and the action of polyene antibiotics, such as amphotericin B and nystatin, including their efficacy, mechanisms of toxicity, and clinical uses. Additionally, the document outlines the azole class of antifungals, their mechanisms of action, resistance patterns, and specific drug examples used for treating various fungal infections.























![▪1-[2-[(4-Chlorophenyl)methoxy]-2-(2,4-
dichlorophenyl)-ethyl]-1H-imidazole
(Spectazole) is a white crystalline nitric acid salt
of econazole. It is only slightly soluble in water
and most organic solvents.
▪Econazole is used as a 1% cream for the topical
treatment of local tinea infections and cutaneous
candidiasis.](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-35-320.jpg)
![▪ 1-[4-(4-Chlorophenyl)-2-[(2,6-dichlorophenyl)-thio]butyl]-1H-
imidazole
▪ An extremely broad-spectrum antifungal drug that is specifically
effective against C. albicans.
▪ It is supplied as a vaginal cream containing 2% of the salt.
▪ It is intended for the treatment of vaginal candidiasis](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-36-320.jpg)
![▪ 1-[2,4-Dichloro-/3-[p-chlorobenzyl)thio]phenethyl]imidazole
mononitrate (Exelderm)
▪ White crystalline nitric acid salt of sulconazole.
▪ It is sparingly soluble in water but soluble in ethanol.
▪ The salt is used in a solution and a cream in 1% concentration for the
treatment of local tinea infections,
▪ such as jock itch, athlete’s foot, and ringworm.](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-37-320.jpg)
![▪ (Z)-1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethanone-
▪ O-[2,4-dichlorophenyl)methyl]oxime mononitrate (Oxistat)
▪ White crystalline nitric acid salt
▪ It is used in cream and lotion dosage forms in 1% concentration for
the treatment of
▪ tinea pedis, tinea corporis, and tinea capitis.](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-38-320.jpg)
![▪ 1-[2-[(2-chloro-3-thienyl)methoxy]2-(2,4-dichlorophenyl)- ethyl]-1H-imidazole
(Vagistat)
▪ Treatment of vulvovaginal candidiasis.
▪ A vaginal ointment containing 6.5% of the free base is available.
▪ Tioconazole is more effective against Torulopsis glabrata than are other azoles.](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-39-320.jpg)
![▪ 1-[2-(2,4-Dichlorophenyl)-2-[2,4-dichlorophenyl]-methoxy]ethyl]-1H-imidazole (Monistat,
Micatin)
▪ Weak base with a pKa of 6.65.
▪ The free base is available in an injectable form (PEG & castor oil) used to treat serious
systemic fungal infections - candidiasis, coccidioidomycosis, cryptococcosis, etc.
▪ Chronic mucocutaneous candidiasis.
▪ Adverse effects serious toxic effects of systemic administration rare but still
thrombophlebitis, pruritus, fever, and GI upset are relatively common.
▪ Dosage forms cream, lotion, powder, and spray (2%) for treatment of tinea infections and
cutaneous candidiasis.
▪ 2%Vaginal creams and suppositories for vaginal candidiasis.](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-40-320.jpg)
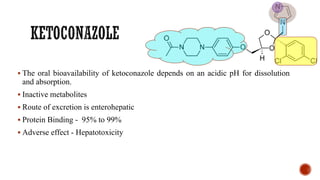
![▪ 1-Acetyl-4-[4-[[2-(2,4-dichlorophenyl)-2(1H-imidazole-1-ylmethyl)-1,3-dioxolan-4-
yl]methoxy]phenyl]piperazine (Nizoral)
▪ A broad-spectrum imidazole antifungal agent
▪ Ketoconazole is a racemic compound, consisting of the cis-2S,4R and cis-2R,4S isomers.
▪ The 2S,4R isomer is 2.5 times more active than its 2R,4S enantiomer.
▪ The trans-isomers, 2S,4S and 2R,4R, are much less active.
▪ Administered orally for the treatment of systemic fungal infections.
▪ It is a weakly basic compound that occurs as a white crystalline solid that is very slightly
soluble in water.](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-41-320.jpg)

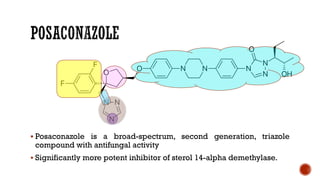
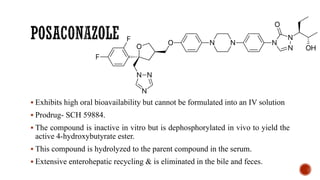
![▪ 4-[4-[4-[4-[[2-(2,4-Dichlorophenyl)-2-1H-1,2,4-triazol-1- ylmethyl)-1,3-dioxolan-4-
yl]methoxy]phenyl]-1-piper- azinyl]phenyl]-2,4-dihydro-2-(1-methylpropyl)-3H-1,2,4-
triazol-3-one (Sporanox)
▪ Unique member of the azole class that contains two triazole moieties in its structure:
❑a weakly basic 1,2,4-triazole and
❑a nonbasic 1,2,4-triazol-3-one.](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-45-320.jpg)

![▪ cis-1-[4-[[2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-yl-methyl)-1,3-
dioxolan-4-yl)methoxy]phenyl]-4-(1-methylethyl)piperazine
(Terazol)
▪ Used exclusively for the control of vulvovaginal moniliasis caused
by C. albicans and other Candida species.
▪ Creams containing 0.4% and 0.8%
▪ Suppositories containing 80 mg of the free base are also available.](https://image.slidesharecdn.com/antifungalagents-200909034610/85/Antifungal-agents-49-320.jpg)