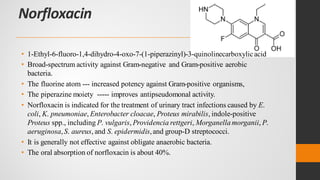
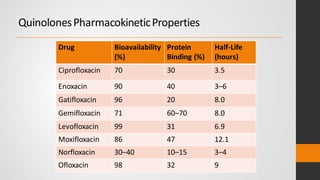
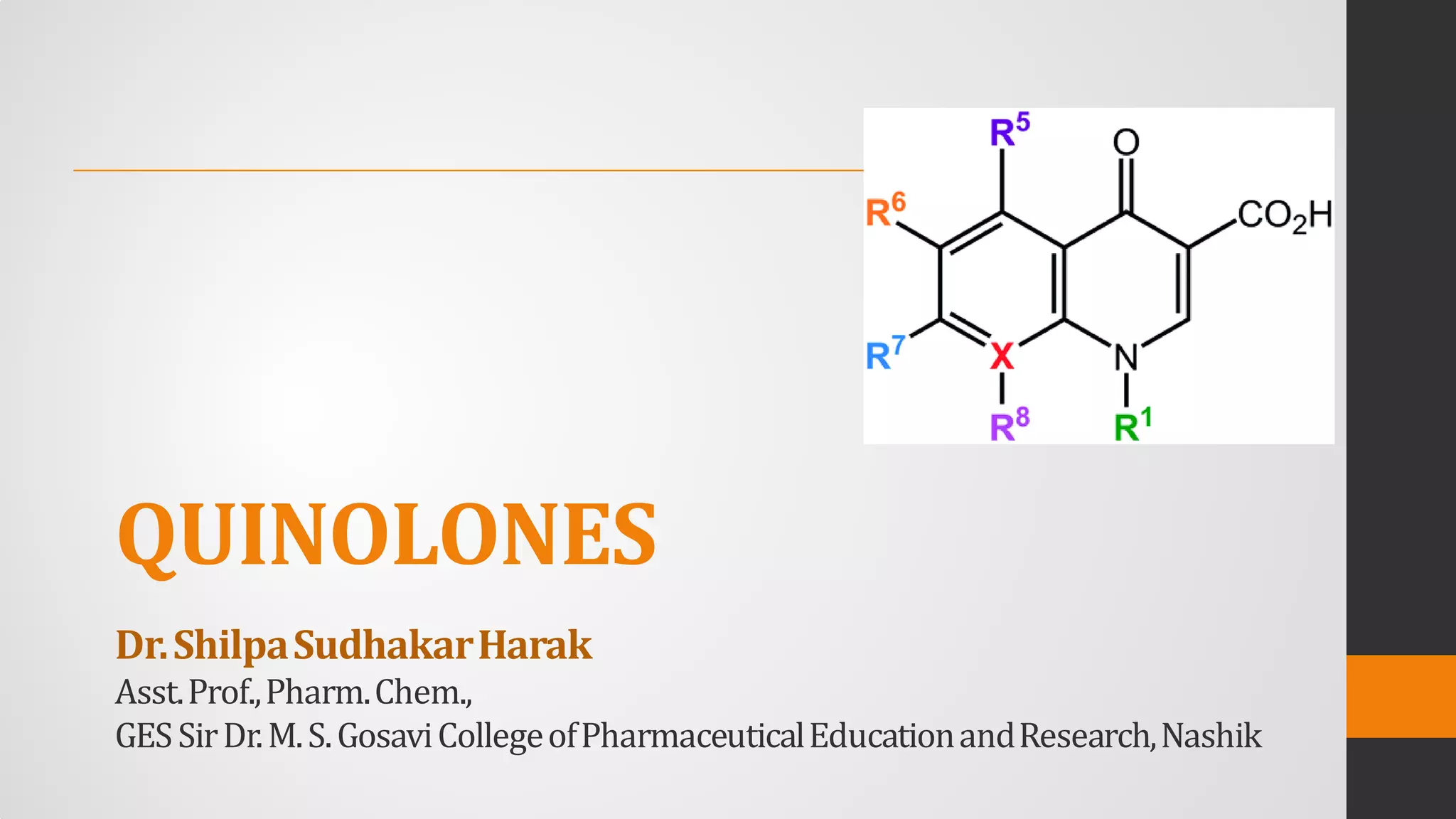
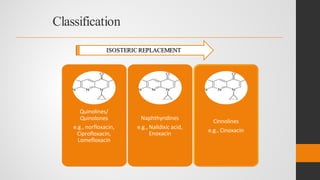
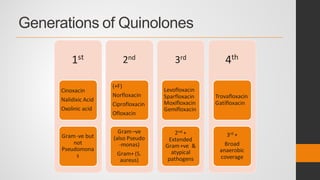
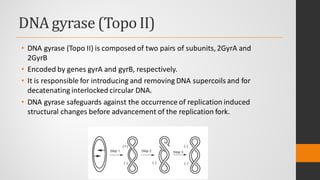
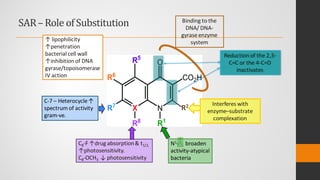
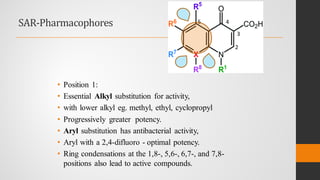
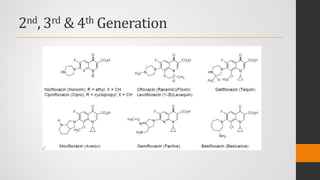
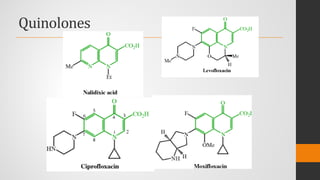
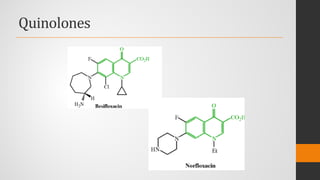
The document provides an overview of quinolones, starting with the lead structure nalidixic acid, and details the classification based on dissociation properties into two main classes. It discusses the mechanisms of action, including inhibition of bacterial DNA gyrase and topoisomerase IV, and describes the evolution of quinolone generations, highlighting specific drugs like ciprofloxacin and norfloxacin. The pharmacokinetics and resistance mechanisms associated with quinolones are also addressed, emphasizing their clinical applications primarily in treating urinary tract infections.






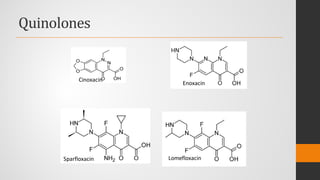
![Cinoxacin
• 1-Ethyl-1,4-dihydro-4-oxo[1,3]dioxolo[4,5g]cinnoline-3-carboxylic
acid (Cinobac) is a close congener (isostere) of oxolinic acid and
• Has antibacterial properties similar to those of nalidixic and oxolinic
acids.
• Urinary tract infections caused by strains of Gram-negative bacteria
• Possesses pharmacokinetic properties superior to those of either of its
predecessors.
• oral administration,
• higher urinary concentrations of cinoxacin than of nalidixic acid or
oxolinic acid are achieved.
• Cinoxacin appears to be more completely absorbed and less protein
• bound than nalidixic acid.](https://image.slidesharecdn.com/quinolones-200825105936/85/Quinolones-23-320.jpg)