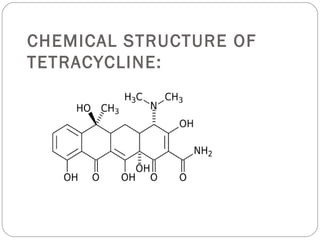
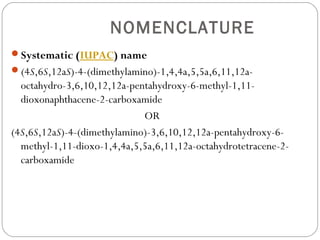
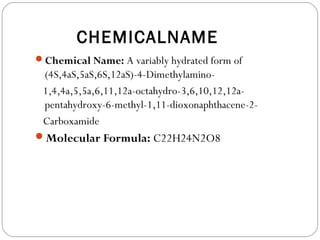
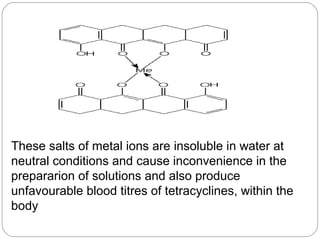
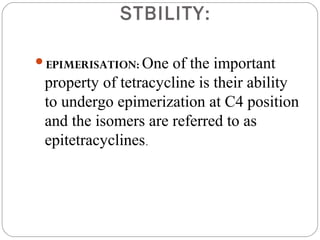
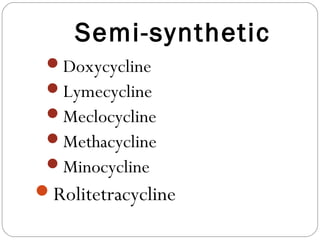
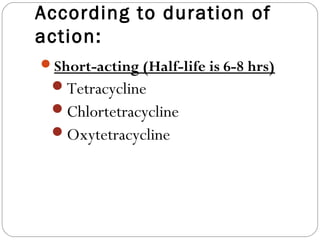
Tetracyclines are a class of antibiotics with a yellow crystalline powder appearance. They are slightly soluble in water but more soluble in alcohol, methyl alcohol, acetone, and dilute acids or bases. Tetracyclines undergo reactions dictated by their complex structure, being sensitive to mild acidic and basic conditions. They can form both water-soluble and insoluble salts. Tetracyclines also chelate with metal ions, forming insoluble complexes that reduce their bioavailability. They are classified based on their source as natural, semi-synthetic, or duration of action.