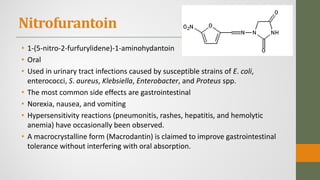
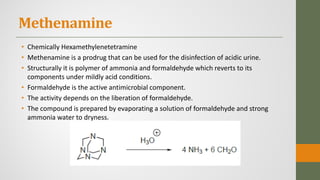
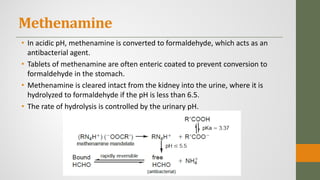
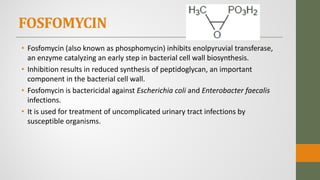
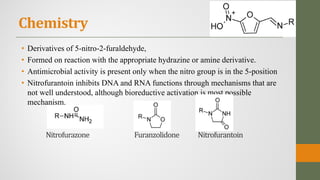
The document discusses nitrofuran compounds used in chemotherapy for bacterial and protozoal infections, focusing on nitrofurazone, furazolidone, nitrofurantoin, and methenamine. It details the mechanisms of action, uses, chemical stability, and common side effects of these drugs, highlighting their effectiveness against various pathogens. Additionally, it mentions methenamine derivatives and fosfomycin, emphasizing their roles in urinary tract infections and their interactions with urine acidity.


![Furazolidone
• 3-[(5-Nitrofurylidene)amino]-2-oxazolidinone (Furoxone)
• Bactericidal activity against a relatively broad range of intestinal pathogens,
including S. aureus, E. coli, Salmonella, Shigella, Proteus spp., Enterobacter,
and Vibrio cholerae.
• It is also active against the protozoan Giardia lamblia.
• Oral treatment of bacterial or protozoal diarrhea caused by susceptible
organisms.
• Approximately 5% of the oral dose is detectable in the urine in the form of
several metabolites.
• Contra-indicated with Alcohol as it can inhibit aldehyde dehydrogenase.](https://image.slidesharecdn.com/nitrofurans-201029101923/85/Nitrofurans-5-320.jpg)